Abstract
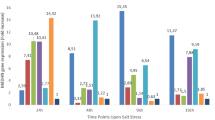
The research was carried out to determine the salt stress tolerance status of some almond genotypes considered as rootstock candidates under in vitro conditions. The genotypes were cultured in the MS nutrient medium containing 0, 50, 100 and 150 mM NaCl. Apart from NaCl, 1.0 mg/l BAP, 0.01 mg/l IBA, 30 g/l sucrose and 7 g/l agar were added to the nutrients and the pH was adjusted to 5.7. In vitro shoots were incubated for 4 weeks in a climate room with 16‑h light and 24 °C temperature, and then the number of shoots per explant, the proline, chlorophyll, total phenolics, total flavonoids and total protein contents, superoxide dismutase, CAT and APX enzyme activities were evaluated. In parallel with the increase in salt stress level, it was determined that the number of shoots and chlorophyll contents decreased significantly in all genotypes as compared to the control treatment. The proline, total phenolic, total flavonoid and total protein contents and enzyme activities increased significantly with the increase in the salt level. In the study, no significant difference was observed regarding the tolerance status of the genotypes in the MS medium containing 50 mM NaCl. Considering the 100 and 150 mM NaCl applications, it was determined that the genotypes numbered 9, 29, 54, 120, 134, 183, 185, 196 and 241 showed better development and therefore they stood out in terms of salt tolerance as compared to the other genotypes.
Similar content being viewed by others
References
Ahmed CB, Rouina BB, Boukhris M (2008) Changes in water relations, photosynthetic activity and proline accumulation in one-year-old olive trees (Olea europaea L. cv. Chemlali) in response to NaCl salinity. Acta Physiol Plant 30:553–560. https://doi.org/10.1007/s11738-008-0154-6
Akça Y, Samsunlu E (2012) The effect of salt stress on growth, chlorophyll content, proline and nutrient accumulation, and K/Na ratio in walnut. Pak J Bot 44(5):1513–1520
Akçay D, Eşitken A (2017) MM106 anacı ve üzerine aşılı Golden Delicious elma çeşidine tuz stresinin etkileri. Selçuk Tarım Bilimleri Dergisi 3(2):228–232
Aono M, Kubo A, Saji H, Tanaka K, Kondo N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol 34(1):129–135. https://doi.org/10.1093/oxfordjournals.pcp.a078386
Aras S, Eşitken A (2018) Physiological responses of cherry rootstocks to short term salinity. Erwerbs-Obstbau 60(2):161–164. https://doi.org/10.1007/s10341-017-0350-x
Aziz I, Khan MA (2001) Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aquat Bot 70(3):259–268. https://doi.org/10.1016/S0304-3770(01)00160-7
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Berli FJ, Moreno D, Piccoli P, Hespanhol-Vıana L, Silva MF, Bressan-Smith R, Cavagnaro JB, Bottini R (2010) Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet‑B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ 33(1):1–10. https://doi.org/10.1111/j.1365-3040.2009.02044.x
Bolat I, Kaya C, Almaca A, Timucin S (2006) Calcium sulfate improves salinity tolerance in rootstocks of plum. J Plant Nutr 29(3):553–564. https://doi.org/10.1080/01904160500526717
Bolat I, Dikilitas M, Ercisli S, Ikinci A, Tonkaz T (2014) The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci World J. https://doi.org/10.1155/2014/769732
Büyük İ, Soydam-Aydın S, Aras S (2012) Bitkilerin stres koşullarına verdiği moleküler cevaplar. Turk Bull Hyg Exp Biol. https://doi.org/10.5505/TurkHijyen.2012.40316
Candan N, Tarhan L (2003) The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Sci 165(4):769–776. https://doi.org/10.1016/S0168-9452(03)00269-3
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35(4):1011–1019. https://doi.org/10.1590/S1415-47572012000600016
Çelik A, Eraslan F (2015) Nitrik oksit uygulamasının tuz stresi altında yetiştirilen mısır bitkisinin mineral beslenmesi ve bazı fizyolojik özellikleri üzerine etkisi. Ziraat Fakültesi Dergisi 10(1):55–64
Çetin ES, Babalık Z, Göktürk Baydar N (2012) Bazı sofralık üzüm çeşitlerinde tanelerdeki toplam karbonhidrat, fenolik madde, antosiyanin, β‑karoten ve C vitamini içeriklerinin belirlenmesi. In: IV. Ulusal Üzümsü Meyveler Sempozyumu, pp 3–5
Channuntapipat C, Sedgley M, Collins G (2003) Micropropagation of almond cultivars Nonpareil and Ne Plus Ultra and the hybrid rootstock Titan x Nemaguard. Sci Hortic 98:473–484. https://doi.org/10.1016/S0304-4238(03)00067-0
Constantine NG, Stanley KR (1977) Superoxide dismutases. Plant Physiol 59(309):e314
Çulha Ş, Çakırlar H (2011) Tuzluluğun bitkiler üzerine etkileri ve tuz tolerans mekanizmaları. Afyon Kocatepe Üniversitesi Fen Ve Mühendislik Bilimleri Dergisi 11(2):11–34
Dal Bİ, Yazıcı K, Baktır İ (2001) Tuz stresi ve bahçe bitkileri üzerindeki etkileri. Derim 18(3):122–131
Daşgan HY, Koç S, Ekici B, Aktaş H, Abak K (2006) Bazı fasulye ve börülce genotiplerinin tuz stresine tepkileri. Alatarım 5(1):23–31
Dat JS, Vandenabeele E, Vranova M, Van Montagu D, Inze F, Breusegem FV (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
De Britto AJ, Gracelin DHS, Sebastian SR (2011) Antibacterial activity of a few medicinal plants against Xanthomonas campestris and Aeromonas hydrophila. J Biopestic 4(1):57
Demiral T, Türkan I (2006) Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ Exp Bot 56(1):72–79. https://doi.org/10.1016/j.envexpbot.2005.01.005
Doğru A, Canavar S (2020) Bitkilerde tuz toleransının fizyolojik ve biyokimyasal bileşenleri. Akademik Platf Mühendislik Ve Fen Bilimleri Dergisi 8(1):155–174. https://doi.org/10.21541/apjes.541620
Dölarslan M, Gül E (2012) Toprak bitki ilişkileri açısından tuzluluk. Türk Bilimsel Derlemeler Dergisi 5(2):56–59
Edrewa A (2005) Generation and scavenging of reactive oxygen species in chloroplasts: a submolecular approaches. Agric Ecosyst Environ 106:119–133. https://doi.org/10.1016/j.agee.2004.10.022
Ertürk U, Sivritepe N, Yerlikaya C, Bor M, Ozdemir F, Turkan I (2007) Responses of the cherry rootstock to salinity invitro. Biol Plant 51(3):597–600
Feng X, Chen L, Lei N, Wang S, Xu X, Zhou G, Li Z (2017) Emulsifying properties of oxidatively stressed myofibrillar protein emulsion gels prepared with (−)-epigallocatechin-3-gallate and NaCl. J Agric Food Chem 65(13):2816–2826. https://doi.org/10.1021/acs.jafc.6b05517
Fu M, Li C, Ma F (2013) Physiological responses and tolerance to nacl stress in different biotypes of malus prunifolia. Euphytica 189:101–109
Gadallah MAA (1999) Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol Plant 42(2):249–257
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Halliwell B (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476(2):107–112. https://doi.org/10.1016/j.abb.2008.01.028t
Hartree EF (1972) Determination of protein: a modification of the lowry method that gives a linear photometric response. Anal Biochem 48(2):422–427. https://doi.org/10.1016/0003-2697(72)90094-2
Hatami E, Shokouhian AA, Ghanbari AR, Naseri LA (2018) Alleviating salt stress in almond rootstocks using of humic acid. Sci Hortic 237:296–302. https://doi.org/10.1016/j.scienta.2018.03.034
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80 % acetone. Plant Physiol 77(2):483–485. https://doi.org/10.1104/pp.77.2.483
Kafi M, Stewart WS, Borland AM (2003) Carbohydrate and proline contents in leaves, roots, and apices of salt-tolerant and salt-sensitive wheat cultivars1. Russ J Plant Physiol 50(2):155–162
Köşkeroğlu S (2006). Tuz ve stresi altındaki mısır (Zea Mays L.) bitkisinde prolin birikim düzeyleri ve stres parametrelerinin araştırılması. Yüksek Lisans Tezi, Muğla Üniversitesi, Fen Bilimleri Enstitüsü.
Li W, Chen M, Wang E, Hu L, Hawkesford MJ et al (2016) Genome-wide analysis of autophagyassociated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genomics 17(1):797
Liu B, Li M, Cheng L, Liang D, Zou Y, Ma F (2012) Influence of rootstock on antioxidant system in leaves and roots of young apple trees in response to drought stress. Plant Growth Regul 67(3):247–256
Mashayekhi M, Amiri ME, Habibi F (2015) Study of the biochemical responses and enzymatic activity of GF677 (peach and almond hybrid) rootstock to in vitro salinity stress. J Hortic Sci 29(2):207–2015
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49(1):69–76. https://doi.org/10.1016/S0098-8472(02)00058-8
Moore JN, Janick J (1996) Fruit breeding: tree and tropical fruits; 2. Vine and small fruits; 3. Nuts. John Wiley & Sons, Oxford
Naik GR, Joshi GV (1983) Ineffectual role of proline metabolism in salt-stressed sugarcane leaves. Proc Plant Sci 92(3):265–269
Najafian SH, Rahemi M, Tavallali V (2008) Effect of salinity on tolerance of two bitter almond rootstock. American Eurasian Journal of Agricultural and Environmental Sciences 3(2):264–268
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Okubo M, Sakuratani T (2000) Effects of sodium chloride on survival and stem elongation of two Asian pear rootstock seedlings. Sci Hortic 85(1):85–90. https://doi.org/10.1016/S0304-4238(99)00141-7
Oluk EA, Sami O (2007) Yüksek öğretim öğrencilerinin sera etkisi, küresel isınma ve iklim değişikliği algılarının analizi. Dokuz Eylül Üniversitesi Buca Eğitim Fakültesi Dergisi 22:45–53.
Öztürk NZ (2015) Bitkilerin kuraklık stresine tepkilerinde bilinenler ve yeni yaklaşımlar. Turk J Agric Sci Technol 3(5):307–315. https://doi.org/10.24925/turjaf.v3i5.307-315.307
Pareek A, Singla SL, Grover A (1997) Salt responsive proteins/genes in crop plants. Oxford, IBH, New Delhi
Paschke M, Bernasconi G, Schmid B (2005) Effects of inbreeding and pollen donor provenance and diversity on offspring performance under environmental stress in the rare plant Cochlearia bavarica. Basic Appl Ecol 6(4):325–338. https://doi.org/10.1016/j.baae.2005.02.005
Ramanjulu S, Sudhakar C (2001) Alleviation of NaCl salinity stress by calcium is partly related to the increased proline accumulation in mulberry (Morus alba L.) callus. J Plant Biol 28:203–206
Ranjbarfordoei A, Samson R, Van Damme P (2006) Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynt 44(4):513–522
Rebey IB, Bourgou S, Rahali FZ, Msaada K, Ksouri R, Marzouk B (2017) Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J Food Drug Anal 25(2):391–402. https://doi.org/10.1016/j.jfda.2016.10.001
Rezazadeh S, Ilkaee M, Aghayari F, Paknejad F, Rezaee M (2019) The physiological and biochemical responses of directly seeded and transplanted maize (Zea mays L.) supplied with plant growth-promoting rhizobacteria (PGPR) under water stress. Iran J Plant Physiol 10(1):3009–3021. https://doi.org/10.22034/IJPP.2019.670787
Scandalios JG (1996) Oxidative stress and moleculer biologoy of antioxidant defenses. Cold Spring Harbor Laboratory, Cold Spring Harbor
Shalaby EE, Epstein E, Qualset CO (1993) Variation in salt tolerance among some wheat and triticale genotypes. J Agron Crop Sci 171(5):298–304. https://doi.org/10.1111/j.1439-037X.1993.tb00144.x
Shibli RA, Shatnawi MA, Swaidat IQ (2003) Growth, osmotic adjustment, and nutrient acquisition of bitter almond under induced sodium chloride salinity invitro. Commun Soil Sci Plant Anal 34(13–14):1969–1979. https://doi.org/10.1081/CSS-120023231
Shiyab SM, Shibli RA, Mohammad MM (2003) Influence of sodium chloride salt stress on growth and nutrient acquisition of sour orange invitro. J Plant Nutr 26(5):985–996. https://doi.org/10.1081/PLN-120020070
Sibley JL, Eakes DJ, Gilliam CH, Keever GJ, Dozier WA, Himelrick D (1996) Foliar SPAD-502 meter values, nitrogen levels, and extractable chlorophyll for red maple selections. HortScience 31(3):468–470. https://doi.org/10.21273/HORTSCI.31.3.468
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Sivritepe N, Erturk U, Yerlikaya C, Turkan I, Bor M, Ozdemir F (2008) Response of the cherry rootstock to water stress induced invitro. Biol Plant 52(3):573
Sorkheh K, Shiran B, Rouhi V, Khodambashi M, Sofo A (2012) Salt stress induction of some key antioxidant enzymes and metabolites in eight Iranian wild almond species. Acta Physiol Plant 34(1):203–213
Sotiropoulos TE, Dimassi KN, Tsirakoglou V, Therios IN (2006) Responses of two Prunus rootstocks to KCl induced salinity invitro. Biol Plant 50(3):477–480
Tilkat EA, Kaplan A, Hoşer A, Tilkat E (2017) In vitro şartlarda yetiştirilen buttum (Pistacia khinjuk Stocks)’da çözünür karbonhidrat değerleri ile antioksidan peroksidaz aktivitesi üzerine tuz stresinin etkileri. Batman Üniversitesi Yaşam Bilimleri Dergisi 7(2/2):90–97
Turfan N (2016) Yerel ceviz çeşidinde (Juglans regia L.) abiyotik stres faktörlerine karşı dayanıklılık mekanizmasının belirlenmesi. Anadolu Tarım Bilimleri Dergisi 31(3):321–331. https://doi.org/10.7161/omuanajas.269984
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V (2014) Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S Afr J Bot 93:92–97. https://doi.org/10.1016/j.sajb.2014.04.002
Yıldırım AN, Tekintaş E, Koyuncu F (2007) Isparta bölgesinde geç çiçeklenen ve üstün nitelikli meyve veren badem (Prunus amygdalus Batsch.) genotiplerinin seleksiyonu. Adnan Menderes Üniversitesi Ziraat Fakültesi Dergisi 4(1–2):39–48
Yıldız M, Terzi H, Cenkci S, Terzi E, Uruşak B (2010) Bitkilerde tuzluluğa toleransın fizyolojik ve biyokimyasal markörleri. Anadolu Univ Sci Technol Life Sci Biotechnol 1(1):33
Yılmaz S, Temizgül R, Yürürdurmaz C, Kaplan M (2020) Oxidant and antioxidant enzyme response of redbine sweet sorghum under NaCl salinity stress. Bioagro 32(1):31–38
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167(3):527–533. https://doi.org/10.1016/j.plantsci.2004.04.020
Zrig A, Tounekti T, Vadel AM, Mohamed HB, Valero D, Serrano M, Chaker C, Habib K (2011) Possible involvement of polyphenols and polyamines in salt tolerance of almond rootstocks. Plant Physiol Biochem 49(11):1313–1322. https://doi.org/10.1016/j.plaphy.2011.08.009
Zrig A, Mohamed HB, Tounekti T, Khemira H, Serrano M, Valeroc D, Vadelet AM (2016) Effect of rootstock on salinity tolerance of sweet almond (cv. Mazzetto). S Afr J Bot 102:50–59. https://doi.org/10.1016/j.sajb.2015.09.001
Acknowledgements
This research is an output of project number 215O779 supported by The Scientific and Technological Research Council of Turkey (TUBITAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.N. Yildirim, B. Şan, F. Yildirim, C. Çelik, B. Bayar and Y. Karakurt declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Yildirim, A.N., Şan, B., Yildirim, F. et al. Determining the Tolerance of Selected Almond Rootstock Genotypes to Salt Stress Under in vitro Conditions. Erwerbs-Obstbau 65, 299–310 (2023). https://doi.org/10.1007/s10341-022-00827-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00827-y