Abstract
The “ecology of fear”, i.e., physiological and behavioral alterations displayed by pests in response to predation risk, has recently been proposed as a sustainable alternative to chemicals for pest control. However, the development of such a strategy requires a detailed understanding of the signals and cues underlying the pest-antagonist interaction and eliciting the prey behavioral alteration. Here, we characterized the substrate-borne vibrations produced during the interaction between the green peach aphid Myzus persicae and its antagonists, the parasitoid wasp Aphidius colemani and the ladybug Adalia bipunctata. Thereafter, coupling the electrical penetration graph (EPG) with a stimulus controller, we evaluated whether the playback of the vibrations, alone and in combination with the alarm pheromone, impacted aphid probing behavior and interaction with the host plant. Aphids responded to vibrations exhibiting longer non-probing, shorter intracellular probes, i.e. the behavior through which the insect evaluates host plant quality, delay in accessing the phloem vessels and decrease of the frequency of phloem salivation events. In contrast, on plants treated with the alarm pheromone, insects displayed longer intracellular probes. We hypothesize that the alarm pheromone, signaling a distant threat, might induce a careful evaluation of the host plant in order to decide the magnitude of the reaction. On the other hand, vibrations might indicate a closely approaching threat pushing the aphid to rush the host evaluation process and the whole feeding process. The possible repercussion of the behavioral alterations observed on the dynamics of aphid-borne plant virus transmission is also discussed.
Similar content being viewed by others
Key message
-
The green peach aphid Myzus persicae is a major agricultural pest and vector of plant viruses.
-
Vibrations produced during aphid-antagonist communication alter aphid-plant interaction.
-
Substrate-borne vibrations might be harnessed in the frame of sustainable aphids control.
Introduction
Aphids are the never-ending challenge of agriculture: prolific, adaptable, colonizers, resistant, often protected by mutualistic associations with ants (Ivens and Kronauer 2022; Depa et al. 2020). These pests can cause considerable damage to crops, either directly, by injuring the plant tissues during their trophic activity, or indirectly, by transmitting viruses capable of seriously compromising agricultural productivity (Dedryver et al. 2010).
To date, aphids control still majorly relies on pesticides, although more and more active substances have been subjected to evaluation and ban by the European supervisory authorities (Regulation (EU) No 485/2013 2013; Regulation (EU) 2018/113 2018; European Food Safety Authority (EFSA) 2013, 2018a, 2018b). The lethal and sublethal effects of some broad-spectrum active substances on pollinators and natural enemies used for biological control are largely reported in scientific literature (Serrão et al. 2022; Panini et al. 2021; Biondi et al. 2012, 2013; Ricupero et al. 2020; Calvo-Agudo et al. 2019; Sánchez-Bayo et al. 2016). Beside their negative impact on beneficial insects and the ecosystem services they provide, pesticides cause environmental degradation, plant physiology alteration (Shahid et al. 2021; Sharma et al. 2019) and, lastly, harm to human health (Nicolopoulou-Stamati et al. 2016; Thompson et al. 2020). In addition, the misuse of pesticides has contributed to the insurgence of aphid populations resistant to most commercially available toxicants (Bass et al. 2014, 2015; Hawkins et al 2019; Panini et al. 2021; Troczka et al. 2021). Overall, these environmental, social and technical concerns call for research on sustainable alternative to chemicals for pest control.
The “ecology of fear”, i.e. physiological and behavioral alterations displayed by an insect in response to the predation risk, has been recently proposed as a new eco-friendly venue to contain phytophagous insect populations below economic thresholds (Pekas et al. 2023). However, the design of bio-inspired control tools and strategies aimed at manipulating pest behavior and disrupt pest-plant interactions exploiting the ecology of fear requires an intimate understanding of the multimodal intra- and inter-specific communication of insects with their antagonists (Polajnar et al. 2015; Nieri et al. 2022; Zapponi et al. 2023; Avosani et al. 2023).
Insects perceive the threat posed by an approaching antagonist through chemical, visual, and vibrational cues. Aphids do not possess tympanal sensory organs and are theoretically not responsive to air-borne vibrations; in contrast, several studies have demonstrated that substrate-borne vibrations, possibly perceived through mechanoreceptors located on the antennae, elicit a wide array of context-dependent behavioral responses (Bromley et al. 1980; Lima and Dill 1990; Humphreys et al. 2021). Substrate-borne vibrations have also been suggested to be a relevant component of natural enemies eavesdropping, as well as intra-specific communication and triggering of collective defenses in aphid colonies (Roitberg and Myers 1978; Clegg and Barlow 1982; Brodsky and Barlow 1986; Francke et al. 2008; Fréchette et al. 2008; Gish 2021; Kubota 1985; McAllister and Roitberg 1987; Losey and Denno 1998; Hartbauer 2010; Yack 2016; Parent et al. 2022).
The perception of the approaching threat is nonetheless assumed not to be only vibrations-based; the latter indeed is deemed to complement other warning cues, as visual cues and alarm pheromones (Vandermoten et al. 2012). While visual cues alert the aphid without triggering a defensive response, strong reactions were reported to the combination of pheromone and vibrations (Roitberg and Myers 1978; Gish 2021). The alarm pheromone alone does not warrant a drastic response by the perceiving aphid (Roitberg and Myers 1978); in addition, cornicle secretion and emission of the alarm pheromone may be less widespread than assumed, given the energetic cost associated to such behavior, with lipids being the main limiting factor for phloem feeders (Alfaress et al. 2018). Therefore, the pheromone could serve as a “sensitizer”, rather than directly work as a warning signal, thus making the aphid more reactive to other warning cues as incidental vibrations.
While previous studies have investigated aphids’ response to generic (i.e., not specific) vibrations (Lee et al. 2012), no information are available on how specific substrate-borne vibrations incidentally produced during aphids’ interaction with natural enemies might affect probing (all the behaviors performed from stylets insertion into the host plant tissues to withdrawal) and feeding (sap ingestion, or in the case of aphids, salivation in phloem and phloem sap ingestion) behaviors. Such behaviors are associated with host plant acceptance and settling, together with the transmission of aphid-borne plant viruses.
If vibrations emitted by foraging antagonists, or by aphids perceiving the antagonist, are important cues underlying aphids’ response to the threat, such vibrations could theoretically be exploited for devising new control strategies alternative to conventional pesticides based on the principle of behavioral manipulation.
Here, we isolated and characterized the substrate-borne vibrations produced during the interaction of the green peach aphid Myzus persicae Sulzer (Aphididae, Hemiptera) with the predator Adalia bipunctata Linnaeus (Coccinellidae, Coleoptera) and the parasitoid Aphidius colemani Viereck (Braconidae, Hymenoptera). Coupling the electrical penetration graph technique (EPG) (McLean and Kinsey 1964; Tjallingii 1978) with a stimulus controller, we furtherly explored the possibility to exploit antagonist-emitted or aphid-emitted vibrations, either played back alone or in combination with the alarm pheromone, to alter aphids probing and feeding behaviors associated with host plant recognition and acceptance, and virus transmission.
Materials and methods
Plants and insects rearing protocol
One-week old dwarf pea plants (Pisum sativum, Pisello a grano rugoso progress n.9, Moon Garden Srl) were used for aphids rearing. Seeds were sown indoor in transparent plastic pots filled with perlite (PERALIT25—Perlite Italiana srl, MI, Italia) after being soaked in water for 30 min.
Myzus persicae colonies were maintained under controlled conditions (21 ± 1 °C, L16:D8, 60 ± 5% RH), in a climatic chamber (Pfeuffer GmbH, Germany) on dwarf pea plants inside 200 ml cups half filled with wet perlite (PERALIT25—Perlite Italiana srl, MI, Italia). Colonies were replaced once a week, transferring pieces of leaves or stem colonized by aphids in newly prepared cups with fresh plants.
Two-weeks old Aphidius colemani males and females (Aphipar500®, Koppert B.V, Netherlands; AphidiPAK500®, Bioplanet Italia) were kept at 24 ± 2 °C, L16:D8, 60 ± 5% RH in 15 × 15 cm plastic cages with net on the lid. Parasitoids were fed with honey and water. Before being released inside the arena used for vibrations recordings (see below), the females were moved to a cup with 3–4 aphids and observed for few minutes; only active females displaying searching behaviors or probing the plant with the ovipositor were used in the experiment; oviposition attempts were prevented by suddenly removing the female from the cage when too close to an aphid.
Adalia bipunctata larvae (Aphidalia 100®, Koppert Italia; Adalia80®, Bioplanet Italia) were kept at 24 ± 2 °C, L16:D8, 60 ± 5% RH in 15 × 15 cm plastic cages filled with cardboard provided only with water until being used in the trial. The cage was filled with pieces of cardboard or sawdust. The predation trial was performed using fourth instar larvae.
Substrate-borne vibrations recording
The trials were carried inside a soundproof chamber (TREBI GmbH), on an antivibration table (Standa LTD). Vibrational cues were recorded using a laser Doppler vibrometer (VibroGo, Polytec GmbH, Germany) focused on the reflective sticker that was placed on the stem of a pea plant (diameter 2–3 mm), approximately 1–2 cm below the apical shoot where aphids were feeding. Recordings were acquired using the software BK Connect (version 2021.1, Hottinger Brüel and Kjær, Nærum, Denmark) with 8 kHz sample rate and 32-bit depth resolution and were stored in the hard drive of a computer connected to the laser through a data acquisition device (LAN-XI type 3050-B-040, Hottinger Brüel and Kjær). In each trial, to associate vibrations and insects’ behaviors, the plant and the insects were filmed using camera (Canon EOS 80D (W), Canon Inc. Japan) with the laser plugged in as external microphone.
We recorded vibrations produced during the interaction with the natural enemies in two conditions: i) single M. persicae parthenogenetic female; ii) one parthenogenetic female together with her progeny. We tested the female with her offspring given that several authors have reported vibrational signaling as important component of anti-predator behaviors in parent-offspring interaction (Cocroft 1996; Ramaswamy and Cocroft 2009; Hamel and Cocroft 2012).
For each recording session, a replicate was prepared 24 h before recording with one adult female of M. persicae on a six-day old dwarf pea plant, in order to acclimate insects to the experimental room and obtain nymphs (0–3 nymphs produced in 24 h).
The reflective sticker was glued on the stem of the plant the day before the recording to avoid any disturbance.
The video-laser recordings had a total duration of 20 min. During the first five minutes, the aphids were alone on the plant inside the arena; thereafter, the natural enemy was released inside the arena and left interacting with the prey/host for the following 15 min. The replicates were 20 for each aphid/antagonist combination (80 recordings in total): 40 recordings for the system M. persicae adult female and predator/parasitoid (20 with A. bipunctata larvae, 20 with A. colemani) and 40 for the system M. persicae adult female plus nymphs and predator/parasitoid (20 with A. bipunctata, 20 with A. colemani).
Videos were analyzed with the software Boris (Friard and Gamba 2016; BORIS (unito.it)) in order to select the behaviors performed by the natural enemies (and the associated vibrations) that triggered a behavioral response in aphids clearly visible in the videos (and the associated vibrations). The analysis consisted in reporting the presence of at least one reaction per aphid to antagonists’ activities. Only the first reaction was considered, regardless of the time required to react.
For the characterization of the vibrations produced during the aphid-antagonist interaction, audio files were analyzed with BK Connect software, with a frequency resolution of 4 Hz, 800 lines, Hanning FFT type and 75% overlap. The peak amplitude of vibrations (as velocity of substrate displacement, mm/s) and the dominant frequency (Hz) were measured. Since the vibrations produced during the interactions were incidental, i.e., derived from insect movement (Strauß et al. 2021), only the recordings with the best signal-to-noise ratio were chosen for characterization.
Impact of vibrations and alarm pheromone on aphid probing and feeding behavior
The aim of this trial was to evaluate if aphids’ probing and insect-plant interaction might be altered by vibrations associated to antagonistic encounters. Since intra-specific communication in aphids is reportedly mediated by alarm pheromones, and we cannot exclude that the response behaviors observed in the behavioral essay were only induced by the pheromone released by tested aphids, we evaluated the effect of vibrations and alarm pheromone either alone or in combination. Using the EPG technique, we analyzed the probing behavior of aphids on one-week old pea plants (the species used for insect rearing and for the behavioral essay) in response to: i) vibrations directly produced by the natural enemy during foraging (A. bipunctata chewing, see results section); ii) vibrations produced by the aphid in response to the natural enemy (M. persicae twitching; see the results section); ii) pheromone alone (E-beta-farnesene); iii) vibration and pheromone combined (chewing + pheromone and twitching + pheromone).
The vibrations were played back on pea plants using linear resonant actuators (LRAs) (9 mm × 3.4 mm; Fyber Labs, Inc., Kirkland, WA, USA) attached with dental wax (Surgident PeripheryWax, Australia) to the stem of the plant. LRAs were connected through an audio interface (AIR 192|14, M-Audio, USA) to a laptop (HP 250 G7) and the audio file was played back in loop with Audacity® for the duration of EPG recordings (6 h). Audio files used for the EPG playback experiment were prepared using Raven Pro 1.6 (The Cornell Lab of Ornithology, Ithaca, NY, USA). Before starting each EPG trial with vibrations, the quality of the playback and the correct functioning of LRAs were tested by recording the vibrations on the plant stem for 10 s with the laser (see results section).
The alarm pheromone (E-beta-farnesene) (or clean air in case of the control; see below) was delivered to each plant with plastic tubes (diameter) connected to the ampoule of the stimulus controller (Stimulus Controller CS 55, © 2018 Ockenfels Syntech GmbH), at an air flow rate of 0.5 L/min. A rubber septum loaded with 10 uL of a 1:10 solution of E-beta-farnesene (Bedoukian Research, Inc USA) and hexane (100 ug of E-beta-farnesene loaded each time) (Cui et al. 2012) was inserted in the ampoule. The rubber septa were previously prepared and kept in freezer until one hour before each pheromone trial.
For the vibrations-alone treatment, the LRAs were turned on and transmitted the signal for the duration of the EPG recording while the stimulus controller was emitting clean air over the plant. For the vibration + pheromone treatment, LRAs were turned on and transmitted the signal while the stimulus controller was emitting the pheromone. For the pheromone-treatment, LRAs were turned off while the stimulus controller was emitting the pheromone. The control consisted of no treatment on the plant with LRAs turned off and clean airflow.
We carried out 20 replicates (twenty 6 h EPG recordings) per each of the five treatments plus the control.
For the EPG, M. persicae apterous adults were collected with a paintbrush from pea plants and starved in a Petri dish for one hour. Afterward, the insects were immobilized with a vacuum device (Aspeed 3.0 3A Healthcare s.r.l.) and connected using a water-based silver glue (EPG-Systems, Wageningen, The Netherlands) to a thin gold wire (18 μm diameter, 3 cm length) glued with an acetone-based silver glue (Ted Pella, INC), to a 2 cm copper wire welded to a brass nail. Aphids were then placed on the pea plants (abaxial portion of an apical leaf) at approximately 10 cm distance from the linear resonant actuators (LRAs). The probing behavior of aphids was monitored for six hours using a Giga-8dd device (EPG Systems, Wageningen, The Netherlands), from 11am to 5 pm. Each replicate corresponded to a single combination of aphid-plant. From six to eight EPG channels were used per day; the treatment applied to each plant was the same for each run for impossibility of conveying different playback files from the same audio interface. Recordings were performed inside a Faraday cage, in an acclimatized room (20 ± 2 °C, 30% RH) provided with artificial illumination. EPG signals were recorded and analyzed using Stylet + software for Windows (EPG Systems, Wageningen, The Netherlands). EPG waveforms were interpreted according to “aphid waveform characteristics” file available on EPG Systems website (Aphid waveform characteristics (epgsystems.eu)) as follows: waveform np (non-probing behavior), C (stylets pathway, i.e. stylets moving intercellularly searching for phloem vessels), pd (potential drop, brief intracellular probes performed to assess the host plant quality), pd-II1, pd-II2, pd-II3 (potential drop subphases, respectively, watery saliva injection and possible egestion, egestion and/or salivation, and active ingestion of cell protoplasm), E1e (extracellular salivation, namely a salivation event during the pathway phase rather than inside phloem vessels), E1 (phloem salivation), E2 (phloem ingestion), G (xylem feeding), waveform F (derailed stylets, namely difficulties in stylets intercellular penetration), phloem-pd (ppd, brief stylets insertion inside phloem vessels or companion cells that are also composed by three subphases—ppd-II1, ppd-II2, ppd-II3—and usually precedes the onset of phloem phase) (Jiménez et al. 2018, 2020a, 2020b, Jiménez Ruiz 2019, ). The implication of these waveforms for the transmission of viruses is summarized in Table 1.
EPG variables were measured following Backus et al. (2007). Per each waveform, the following non-sequential variables were calculated and compared: i) number of waveform events per insect (NWEI) (namely, the sum of the waveform events during the 6 h recording); ii) waveform duration (WDI) (the sum of duration of a particular waveform events during the 6 h recording); iii) waveform duration per event per insect (WDEI) (the average duration of a single waveform event), iv) proportion of individuals that produced a specific waveform (PPW), v) total probing time. The sequential variables considered were: i) time to first probe from the start of the recording; ii) time from the beginning of the first probe to first pd; iii) time from start of EPG to first E1; iv) time from first probe to first E1; v) time from start of EPG to first E2; vi) time from start of EPG to first sustained E2 (longer than 10 min); vii) time from first probe to first E2; viii) time from first probe to first sustained E2; ix) time from start of EPG to first phloem-pd; x) time from the beginning of first probe to first phloem-pd; xi) and time from first phloem-pd to first E2. The proportion of individuals producing a specific waveform (PPW) was also analyzed. EPG variables were processed using the automatic EPG-Excel Data Workbook developed by Sarria et al. (2009).
Statistical Analyses
Statistical analyses were carried out using R, version 4.1.0 (R Core Team, 2021). Probing behavioral differences between the treatments and the control (i.e., sequential and non-sequential EPG variables) were investigated with a linear mixed-effect model (LME), using the control as model baseline; the dates of the EPG recordings were included as random effect. The EPG data were transformed with ln (x + 1) or √ (x) to reduce heteroscedasticity and improve normal distribution. Models were run using the “glmmTMB” package (Magnusson et al. 2017), while residual distribution was checked using the “DHARMa” package (Hartig and Hartig 2021). In case of a statistically significant effect (P < 0.05) of model variables, pairwise comparison was conducted by Tukey’s HSD (honest significant differences) test using “emmeans” package (Lenth 2021).
Bar charts were generated using the “ggplot2” package.
Results
Behavioral response of aphids to the antagonists
After their release inside the arena, antagonists displayed a series of behaviors that can be divided in behaviors not involving physical contact (walking and foraging) and behaviors involving physical contact (touching between individuals; oviposition attempts by the parasitoid; predator chewing a prey) with the prey/host.
All the behavioral responses of aphids clearly visible in the videos and the related triggering behaviors performed by the antagonists are reported in Tables 2, 3 and in Fig. 1.
The following main response behaviors displayed by the aphids in reaction to the antagonists, or by conspecifics in response to aphids’ reacting to the antagonists, were identified: i) twitching and kicking response; ii) escape (as walking away or dropping of the plant); iii) rubbing. Twitching and kicking, hereafter “twitching”, consisted in a vigorous movement of the abdomen accompanied by forceful kicks with one or both hind legs, without stylet withdrawal (Hartbauer 2010). Whereas, walking and dropping off the plant involved stylets withdrawal. A rubbing event consisted in the aphid continuously rubbing the plant with the legs after a disturbance (Kubota 1985).
Only five aphids out of the total responded to natural enemies walking over the plant; in particular, no reaction to the parasitoid walk was ever observed.
Adalia bipunctata larvae chewing either adults or nymphs elicited a response in about 70% of the observed aphids surrounding the attacked ones, which reacted mainly by walking away from the larvae (48,5%), and secondarily by rubbing (15,2%). In 16 recordings (N = 20, adult M. persicae with nymphs), oviposition attempts by the parasitoid on the adult female were observed, causing the aphid to immediately react defensively by twitching. Simultaneously, 67% of the nymphs reacted to the mother twitching by moving away (25%), twitching themselves (22,2%), or dropping off the plant (13,9%).
Substrate-borne characterization of triggering behaviors
Given that ladybug chewing and aphid twitching were the main behaviors apparently triggering aphids’ response, vibrations associated to chewing and twitching were characterized and used in the EPG trial.
For the twitching vibrations, we analyzed 20 sections of 10 s from 20 trials and 44 single pulses obtained by 17 trials; for chewing vibrations, we analyzed 20 sections of 10 s each obtained by 16 trials (Figs. 2, 3). For the EPG-playback experiment, one chewing selection of 10 s and one of twitching of 30 s were selected to be played back in loop.
The average (± SD) dominant frequency (Hz) and amplitude (mm/s) for the signals were: i) chewing selections: 124.20 ± 54.23 Hz and 2.52 ± 5.08 mm/s; ii) twitching selections: 159.20 ± 75.67 Hz and 0.0033 ± 0.0041 mm/s; iii) twitching pulses: 218 ± 108.15 Hz and 0.0099 ± 0.0156 mm/s.
The chewing selection used in the EPG-playback experiment was characterized by dominant frequency and amplitude of 112 Hz and 0.00141 mm/s, respectively, while 80 Hz and 0.00455 mm/s (with a second highest peak at 460 Hz, 0.00391 mm/s) were the frequency and amplitude of the twitching selection (Figs. 2, 3).
Playbacks were verified before the beginning of each 6 h EPG recording round with airflow on. The average dominant frequency and amplitude among the chewing playbacks were 203.20 ± 149.32 Hz and 0.00475 ± 0.00661 mm/s, respectively, while in twitching playbacks were 379.66 ± 99.16 Hz and 0.05572 ± 0.07613 mm/s.
Vibrations and pheromone impact on aphids probing and feeding behavior
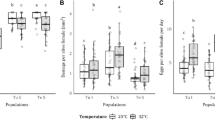
The aphids spent a significantly longer time with stylets out of the plant tissues (non-probing activity; np WDI) on plants treated with the chewing vibration compared to the control (z = 2.474; p = 0.0134). The difference with the other treatments was non-statistically significant (Fig. 4).
Bar charts showing the total duration of a certain probing behavior (WDI) performed by the aphid per treatment over the 6 h EPG recording. C = pathway; E1 = phloem salivation; E2 = phloem ingestion; F = stylets derailment (difficulties in stylets intercellular penetration within plant tissues); G = xylem ingestion; np = non probing (stylets out of the plant); pd = potential drop (intracellular puncture); ppd = phloem pd (intracellular punctures in sieve elements and companion cells). Significance codes (statistically significant differences between treatment and control according to LME): 0; ‘***’ 0.001; ‘**’ 0.01; ‘*’ 0.05; ‘.’ 0.1; ‘‘ 1
The single intracellular punctures (pd WDEI) were significantly longer on plants treated either with pheromones alone (t = 3.198, p = 0.021), or with pheromone coupled with both chewing (t = 3.103, p = 0.0285), and twitching (t = 3.122, p = 0.0270) compared to the control. The differences between pheromone + twitching and twitching alone (t = 4.225, p < 0.001) and pheromone + chewing and chewing alone (t = 3.209, p = 0.021) were statistically significant. Additionally, the duration of the single cell protoplasm ingestion events (pd II-3 WDEI) of the intracellular punctures (pd) was significantly shorter on plants treated with the twitching compared to pheromone alone (t = 4.480, p < 0.001), pheromone + twitching (t = 4.017, p = 0.001), and pheromone + chewing (t = 3.619, p = 0.006).
On plants treated with the twitching vibration, M. persicae exhibited a shorter total duration of the single intracellular salivation events (pd II1 WDEI) compared to both control plants (t = 3.854; p = 0.002) and the other treatments (chewing (t = 3.089, p = 0.029); pheromone (t = 5.106, p < 0.001), pheromone + twitching (t = 5.590, p < 0.001); pheromone + chewing (t = 5.553, p < 0.001) (Fig. 5).
Bar charts showing the average duration of the single waveform events (WDEI) for the potential drops (pds) and the three pd subphases (II-1: watery saliva injection and possible egestion; II-2: egestion and/or salivation; II-3: active ingestion of cell protoplasm). Significance codes (statistically significant differences between treatment and control according to LME): 0; ‘***’ 0.001; ‘**’ 0.01; ‘*’ 0.05; ‘.’ 0.1; ‘‘ 1
Twitching playback also significantly shortened the overall duration of the ingestion of cell protoplasm (pd II3 WDI) compared to control (t = 3.175, p = 0.0231) and other treatments (chewing (t = 3.618, p = 0.006); pheromone (t = 3.373, p = 0.012), pheromone + twitching (t = 3.190, p < 0.022); pheromone + chewing (t = 3.530, p = 0.008).
The combination of pheromone and twitching induced an increase in the number (ppd NWEI) and duration (ppd WDEI) of the brief penetration of phloem and companion cells (ppd) compared to the control (ppd NWEI: z = 2.081, p = 0.037; ppd WDEI: t = 2.740, p = 0.0797). The phloem intracellular punctures events (ppd WDEI) were significantly longer on plants treated with the combination pheromone + twitching compared to twitching alone (t = 3.482; p = 0.011).
The proportion of aphids displaying stylets derailment events (F waveform PPW), indicating difficulties in the intercellular stylets penetration, was higher on plants treated with the twitching vibrations compared to control plants (z = 2.034, p = 0.042), but not significantly different from the other treatments.
The proportion of individuals (PPW) performing watery salivation phase (E1) on plants treated with chewing vibrations was lower compared to control (z = − 2.061; p = 0.039), but similar to the other treatments. Moreover, on plants treated with chewing (z = 2.338; p = 0.019) and twitching (z = 2.249; p = 0.024), the aphids required a longer time to perform the first phloem salivation event compared to the control. For a summary of the EPG results, see Tables 4, 5.
Discussion
In this study we investigated how substrate-borne vibrations emitted during the aphid-antagonist interaction, alone or in combination with the alarm pheromone, may impact the probing behavior of M. persicae. Two were the substrate-borne vibrations associated with behaviors that elicited a response in the aphids: the vibration produced by the ladybug A. bipunctata while chewing the prey, and the twitching associated with the aphid response (abdominal twitching and kicking) to the oviposition attempt by the parasitoid A. colemani.
When the two vibrations, coupled or not with the alarm pheromone, were played back on pea plants, we observed that: i) in response to the chewing vibration alone, aphids tended to spend longer time with the stylets out of the host plant tissues compared to control plants; ii) on plants treated with both chewing and twitching, aphids took more time to engage with phloem salivation (E1). Additionally, with the chewing playback, the proportion of individuals performing phloem salivation events was significantly lower than in the control; iii) the playback of the twitching vibration triggered more frequent stylets derailment events (waveform F) and caused a significant shortening of the intracellular salivation and protoplasm ingestion; iv) in contrast, aphids exposed to the alarm pheromone, both alone or in combination with the vibrations, tended to perform longer intracellular probes events (pd); v) the combination pheromone + twitching triggered more and longer quick penetrations of phloem and companion cells (ppds).
In our experiments, chewing vibrations induced a “freezing” response, causing the aphid to remain still with stylets out of the plant (without probing) for a time significantly longer than in control plants. Remaining still is one of the most common prey responses to incidental vibrations produced by a potential enemy, possibly to avoid the production of vibrational cues that could be eavesdropped revealing the prey’s location (Meyhöfer et al. 1997; Djemai et al. 2001; Kojima et al. 2012). Therefore, the chewing vibration could represent for the aphid an immediate strong warning cues of a closely located menace to which the individual responds by interrupting probing activities and possibly getting ready to escape. Additionally, the playback of the chewing and the twitching vibrations interfered with the phloem phase, with aphids on plants treated with vibrations showing a delayed access to phloem vessels and salivation onset. Furthermore, chewing significantly affected the proportion of individuals performing phloem salivation (E1 waveform). The alteration of the phloem-related activities in response to vibrations derived from aphid-antagonist interactions could have relevant effects on the transmission of persistent phloem limited viruses (Jiménez et al. 2021a, 2021b) and deserve further dedicated investigations.
In contrast, rather than causing a prolonged cessation of the probing activity, the alarm pheromone stimulated in the exposed aphids a more careful evaluation of the host plant quality through longer intracellular probes, independently of vibrations. The vibrations played no role in this behavioral alteration, indicating that longer intracellular probes and host tasting/testing is dependent on the perception of the alarm pheromone alone. The host plant quality indeed might determine the magnitude of the aphid response to the warning cue, with more costly defensive strategies, as abandoning the plant, occurring only in harsher environments and on poor quality hosts (Lima and Dill 1990). No other patterns related to probing and feeding by the green peach aphid were significantly affected by the pheromone, at least on pea plants and under our experimental conditions. Therefore, the alarm pheromone, warning the aphid about a “distant” approaching threat, possibly elicits a more accurate assessment of the host plant; if the host plant quality matches aphid requirements, the individual will remain on the plant and engage in probing and feeding activity independently on the warning cue. On the other hand, longer potential drops, and specifically longer II-3 subphases and associated pulses, are putatively associated with higher transmission efficiency of non-persistent viruses (Collar and Fereres 1998). Therefore, the aphid behavioral response to the alarm pheromone might significantly impact the dynamics of non-persistent viruses transmission; overall, our findings call for dedicated investigations exploring how antagonist-vector interaction may shape the epidemiology of vector-borne plant pathogens, focusing on infective vector response to multiple warning cues in terms of transmission efficiency.
Vibrations possibly convey a message different from the one conveyed by the pheromone, warning the aphid about a closely approaching threat to which the insect respond by hurrying the host evaluation process and disturbing the phloem phase. The playback of the twitching vibrations indeed caused a significant decrease of the duration of the intracellular salivations (II-1) and ingestions (II-3). Moreover, both chewing and twitching vibrations caused a delay in the onset of watery phloematic salivation, probably related to the longer non-probing time with chewing, and the higher frequency of stylets derailment (F) patterns with twitching. Our findings about probing and feeding activities impairment in response to cues associated with an approaching predator are consistent with previous observations on leafhoppers, which similarly exhibit delayed and less frequent feeding when exposed to predation risk (Tholt et al. 2018; Beleznai et al. 2015). Overall, we suggest this delay in the onset of trophic activities is part of a defensive strategy, as inserting the stylets and engaging with phloem phase expose the insect to predation and delay defensive responses. Indeed, stylets withdrawal during phloem ingestion would result in considerable efforts and in delayed escape for the prey; therefore, the presence of a predator, or in our case the perception of vibrational cues evoking an imminent predation, increase insect alertness and decrease the insect-plant interactions. This non-consumptive effect evoked by antagonist cues as vibrations might have relevant implications for the pest population dynamic in the long-term, which deserve further investigation.
Furthermore, our findings are also consistent with previous observations by Lee et al. (2012), who reported a significant alteration of phloem-related activities in M. persicae upon exposure to acoustic stimuli at 100 and above 1000 Hz. In fact, the recorded amplitude peak of our chewing and twitching vibrations, both altering the phloem phase, occurred at 100 Hz (Figs. 2, 3). We suggest that this behavioral alteration was caused by the substrate-borne component of the acoustic stimulus (Seok et al. 2010; Lee et al. 2012; Caldwell 2014).
The longer stylets derailment patterns observed in aphids on plants “treated” with the twitching vibration, rather than representing a real difficulty in stylets penetration caused by the playback, might just have resulted from the individuals starting twitching after sensing the incidental vibrations produced by conspecifics twitching. Indeed, during the twitching the stylets remain within the host plant tissues serving as anchorage while the individual twitches and kicks in response to the signaling of conspecifics threatened by a parasitoid (Hartbauer 2010); the anchorage for twitching may generate the stylets derailment pathway.
The only apparent “synergistic” effect observed coupling vibrations and pheromone was the higher number and longer duration of quick stylets insertion in phloem vessels and companion cells (phloem-pds), that aphids perform to assess the phloem characteristics before engaging with sustained salivation and ingestion. One explanation to such results could be that this alteration of the “phloem tasting” pattern is an artifact triggered by the perception of two contrasting information that alone would normally convey different messages (the pheromone induces a longer host screening process, while the twitching hurries it up). Alternatively, consistently with previous data, the alarm pheromone, rather than directly eliciting drastic responses to the threat, makes aphids more sensitive and prone to react to other warning cues as substrate-borne vibrations (Montgomery and Nault 1978; Clegg and Barlow 1982). Therefore, the two messages combined represents possibly a strong warning inducing the aphid to carefully assess the characteristics of the phloem sap before engaging with an activity as sustained feeding that would make them more vulnerable to antagonists. In this way, a deeper evaluation of the plant is achieved before deciding the magnitude of the reaction to the threat.
Independently of the underlying biological explanation, phloem-pds are associated with the transmission of semi-persistent phloem restricted viruses, thus the playback of twitching combined with the release of pheromone could have a strong impact on the aphid-borne plant viruses epidemiology (Collar and Fereres 1998; Jiménez et al. 2020a, 2020b). The absence of other effects to E-beta-farnesene was maybe due to the habituation of the aphid to the alarm pheromone, and a sensitization to further warning stimuli.
We cannot exclude that the activation of plant defenses in response to substrate-borne vibrations could have also been a component of the alteration of probing and feeding behavioral patterns observed in the present study; further research is needed to investigate the impact of vibrations intended to be used in pest control on the plant phenotype (Appel and Cocroft 2014; Virant-Doberlet et al. 2019).
Overall, our data show that the playback of incidental vibrations produced during the aphid-antagonist interaction bear the potential to alter the aphid-plant interaction. The vibrational signals, in addition to the direct impact on the aphid, might have a major impact even on the epidemiology of vector-borne plant pathogens.
Further studies should expand on our findings investigating i) the impact of such vibrations on other host plant-aphid species systems and ii) on the bionomic of both aphids and beneficial insects, besides iii) the possibility to integrate vibrations with other sustainable control tools as biopesticides.
Author contributions
DC, RN, GA, VM contributed to the study conception and design. Material preparation, data collection and analysis were performed by CZ and ZH. GT, VV, VM, GA and DC provided fundings. The first draft of the manuscript was written by CZ and DC; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Further data are available from the corresponding author (daniele.cornara@uniba.it) upon request. Videos and EPG data (either the recordings or the marked files) are available at Zenodo (the link will be made available upon manuscript acceptance).
References
Alfaress S, Brodersen CR, Ammar ED, Rogers ME, Killiny N (2018) Laser surgery reveals the biomechanical and chemical signaling functions of aphid siphunculi (cornicles). PLoS ONE 13(10):e0204984. https://doi.org/10.1371/journal.pone.0204984
Appel HM, Cocroft RB (2014) Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175(4):1257–1266. https://doi.org/10.1007/s00442-014-2995-6
Avosani S, Nieri R, Mazzoni V, Anfora G, Hamouche Z, Zippari C, Cornara D (2023) Intruding into a conversation: how behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J Pest Sci. https://doi.org/10.1007/s10340-023-01631-7
Backus EA, Cline AR, Ellerseick MR, Serrano MS (2007) Lygus hesperus (Hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of nonsequential electrical penetration graph data. Ann Entomol Soc Am 100(2):296–310. https://doi.org/10.1603/0013-8746(2007)100[296:LHHMFO]2.0.CO;2
Bass C, Puinean AM, Zimmer CT, Denholm I, Field LM, Foster SP, Williamson MS (2014) The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem Mol Biol 51:41–51. https://doi.org/10.1016/j.ibmb.2014.05.003
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 121:78–87. https://doi.org/10.1016/j.pestbp.2015.04.004
Beleznai O, Tholt G, Tóth Z, Horváth V, Marczali Z, Samu F (2015) Cool headed individuals are better survivors: non-consumptive and consumptive effects of a generalist predator on a sap feeding insect. PLoS ONE 10(8):e0135954. https://doi.org/10.1371/journal.pone.0135954
Biondi A, Mommaerts V, Smagghe G, Vinuela E, Zappala L, Desneux N (2012) The non-target impact of spinosyns on beneficial arthropods. Pest Manag Sci 68(12):1523–1536. https://doi.org/10.1002/ps.3396
Biondi A, Zappalà L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 8(9):e76548. https://doi.org/10.1371/journal.pone.0076548
Brodsky LM, Barlow CA (1986) Escape responses of the pea aphid, Acyrthosiphon pisum (Harris)(Homoptera: Aphididae): influence of predator type and temperature. Can J Zool 64(4):937–939. https://doi.org/10.1139/z86-142
Bromley AK, Dunn JA, Anderson M (1980) Ultrastructure of the antennal sensilla of aphids: II. Trichoid, chordotonal and campaniform sensilla. Cell Tissue Res 205:493–511. https://doi.org/10.1007/BF00232289
Caldwell MS (2014) Interactions between airborne sound and substrate vibration in animal communication. Studying vibrational communication. Springer, Heidelberg, pp 65–92. https://doi.org/10.1007/978-3-662-43607-3_6
Calvo-Agudo M, González-Cabrera J, Picó Y, Calatayud-Vernich P, Urbaneja A, Dicke M, Tena A (2019) Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc Natl Acad Sci 116(34):16817–16822. https://doi.org/10.1073/pnas.1904298116
Clegg JM, Barlow CA (1982) Escape behaviour of the pea aphid Acyrthosiphon pisum (Harris) in response to alarm pheromone and vibration. Can J Zool 60(10):2245–2252. https://doi.org/10.1139/z82-289
Cocroft RB (1996) Insect vibrational defence signals. Nature 382(6593):679–680. https://doi.org/10.1038/382679a0
Collar JL, Fereres A (1998) Nonpersistent virus transmission efficiency determined by aphid probing behavior during intracellular punctures. Environ Entomol 27(3):583–591. https://doi.org/10.1093/ee/27.3.583
Cui LL, Dong J, Francis F, Liu YJ, Heuskin S, Lognay G, Liu Y (2012) E-β-farnesene synergizes the influence of an insecticide to improve control of cabbage aphids in China. Crop Prot 35:91–96. https://doi.org/10.1016/j.cropro.2012.01.003
Dedryver CA, Le Ralec A, Fabre F (2010) The conflicting relationships between aphids and men: a review of aphid damage and control strategies. CR Biol 333(6–7):539–553. https://doi.org/10.1016/j.crvi.2010.03.009
Depa Ł, Kaszyca-Taszakowska N, Taszakowski A, Kanturski M (2020) Ant-induced evolutionary patterns in aphids. Biol Rev 95(6):1574–1589. https://doi.org/10.1111/brv.12629
Djemai I, Casas J, Magal C (2001) Matching host reactions to parasitoid wasp vibrations. Proc R Soc Lond Ser B Biol Sci 268(1484):2403–2408. https://doi.org/10.1098/rsbp.2001.1811
European Food Safety Authority (EFSA) (2013) Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid. EFSA J 11(1):3068. https://doi.org/10.2903/j.efsa.2013.3068
European Food Safety Authority (EFSA) (2018a) Peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering the uses as seed treatments and granules. EFSA J 16(2):e05178. https://doi.org/10.2903/j.efsa.2018.5178
European Food Safety Authority (2018) Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU, vol 15, no 2, p 1378. https://doi.org/10.2903/sp.efsa.2018.EN-1378
Fereres A, Moreno A (2009) Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res 141(2):158–168. https://doi.org/10.1016/j.virusres.2008.10.020
Francke DL, Harmon JP, Harvey CT, Ives AR (2008) Pea aphid dropping behavior diminishes foraging efficiency of a predatory ladybeetle. Entomol Exp Appl 127(2):118–124. https://doi.org/10.1111/j.1570-7458.2008.00678.x
Fréchette B, Larouche F, Lucas E (2008) Leucopis annulipes larvae (diptera: chamaemyiidae) use a furtive predation strategy within aphid colonies. Eur J Entomol 105(3):399. https://doi.org/10.14411/eje.2008.050
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7(11):1325–1330. https://doi.org/10.1111/2041-210X.12584
Gish M (2021) Aphids detect approaching predators using plant-borne vibrations and visual cues. J Pest Sci 94(4):1209–1219. https://doi.org/10.1007/s10340-020-01323-6
Hamel JA, Cocroft RB (2012) Negative feedback from maternal signals reduces false alarms by collectively signalling offspring. Proc R Soc B: Biol Sci 279(1743):3820–3826. https://doi.org/10.1098/rspb.2012.1181
Hartbauer M (2010) Collective defense of Aphis nerii and Uroleucon hypochoeridis (Homoptera, Aphididae) against natural enemies. PLoS ONE 5(4):e10417. https://doi.org/10.1371/journal.pone.0010417
Hartig F and Hartig MF (2021) Package ‘DHARMa’. R package version 0.3, 3
Hawkins NJ, Bass C, Dixon A, Neve P (2019) The evolutionary origins of pesticide resistance. Biol Rev 94(1):135–155. https://doi.org/10.1111/brv.12440
Humphreys RK, Ruxton GD, Karley AJ (2021) Drop when the stakes are high: adaptive, flexible use of dropping behaviour by aphids. Behaviour 158(7):603–623. https://doi.org/10.1163/1568539X-bja10083
Ivens AB, Kronauer DJ (2022) Aphid-farming ants. Curr Biol 32(15):R813–R817. https://doi.org/10.1016/j.cub.2022.06.072
Jiménez J, Tjallingii WF, Moreno A, Fereres A (2018) Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited beet yellows virus. J Virol 92(21):10–1128. https://doi.org/10.1128/jvi.01076-18
Jiménez J, Arias-Martín M, Moreno A, Garzo E, Fereres A (2020a) Barley yellow dwarf virus can be inoculated during brief intracellular punctures in phloem cells before the sieve element continuous salivation phase. Phytopathology 110(1):85–93. https://doi.org/10.1094/PHYTO-07-19-0260-FI
Jiménez J, Garzo E, Alba-Tercedor J, Moreno A, Fereres A, Walker GP (2020b) The phloem-pd: a distinctive brief sieve element stylet puncture prior to sieve element phase of aphid feeding behavior. Arthropod-Plant Interact 14:67–78. https://doi.org/10.1007/s11829-019-09708-w
Jiménez J, Moreno A, Fereres A (2021a) Semipersistently transmitted, phloem limited plant viruses are inoculated during the first subphase of intracellular stylet penetrations in phloem cells. Viruses 13(1):137. https://doi.org/10.3390/v13010137
Jiménez J, Moreno A, Fereres A (2021b) Transmission of phloem-limited viruses to the host plants by their aphid vectors. Progress in Bot 82:357–382. https://doi.org/10.1007/124_2020_47
Jiménez Ruiz, J (2019) Non-circulative virus transmission by aphids: new insights into the mechanisms of transmission and the interference for retention sites in the vector (doctoral dissertation, Agronomica). https://doi.org/10.20868/UPM.thesis.55678
K. Lisa Yang (2022) Center for conservation bioacoustics at the cornell lab of ornithology
Kojima W, Ishikawa Y, Takanashi T (2012) Deceptive vibratory communication: pupae of a beetle exploit the freeze response of larvae to protect themselves. Biol Let 8(5):717–720. https://doi.org/10.1098/rsbl.2012.0386
Kubota S (1985) Rubbing behaviours in some aphids. 昆蟲 53(4):595–603
Lee Y, Kim H, Kang TJ, Jang Y (2012) Stress response to acoustic stimuli in an aphid: a behavioral bioassay model. Entomol Res 42(6):320–329. https://doi.org/10.1111/1748-5967.12000
Lenth RV (2021) emmeans: Estimated marginal means, aka least-squares means. R package version 1.6.0., from https://CRAN.R-project.org/package=emmeans
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68(4):619–640. https://doi.org/10.1139/z90-092
Losey JE, Denno RF (1998) The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23(1):53–61. https://doi.org/10.1046/j.1365-2311.1998.00102.x
Magnusson A, Skaug H, Nielsen A, Berg C, Kristensen K, Maechler M, van Bentham K, Bolker B, Brooks M, Brooks MM (2017) Package ‘glmmtmb.’ R Packag Version 02 0, 25. https://doi.org/10.32614/RJ-2017-066
Martin B, Collar JL, Tjallingii WF, Fereres A (1997) Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol 78(10):2701–2705. https://doi.org/10.1099/0022-1317-78-10-2701
McAllister MK, Roitberg BD (1987) Adaptive suicidal behaviour in pea aphids. Nature 328(6133):797–799. https://doi.org/10.1038/328797b0
McLean DL, Kinsey MG (1964) A technique for electronically recording aphid feeding and salivation. Nature 202(4939):1358–1359. https://doi.org/10.1038/2021358a0
Meyhöfer R, Casas J, Dorn S (1997) Vibration–mediated interactions in a host–parasitoid system. Proc R Soc Lond Ser: B Biol Sci 264(1379):261–266. https://doi.org/10.1098/rspb.1997.0037
Montgomery ME, Nault LR (1978) Effects of age and wing polymorphism on the sensitivity of Myzus persicae to alarm pheromone. Ann Entomol Soc Am 71(5):788–790. https://doi.org/10.1093/aesa/71.5.788
Moreno A, Tjallingii WF, Fernandez-Mata G, Fereres A (2012) Differences in the mechanism of inoculation between a semi-persistent and a non-persistent aphid-transmitted plant virus. J Gen Virol 93(3):662–667. https://doi.org/10.1099/vir.0.037887-0
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L (2016) Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health 4:148. https://doi.org/10.3389/fpubh.2016.00148
Nieri R, Anfora G, Mazzoni V, Rossi Stacconi MV (2022) Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. Entomol Generalis 42(2):167–183. https://doi.org/10.1127/entomologia/2021/1236
Panini M, Chiesa O, Troczka BJ, Mallott M, Manicardi GC, Cassanelli S, Bass C (2021) Transposon-mediated insertional mutagenesis unmasks recessive insecticide resistance in the aphid Myzus persicae. Proc Natl Acad Sci 118(23):e2100559118. https://doi.org/10.1073/pnas.2100559118
Parent JP, Laidlaw R, Abram PK (2022) Disruptive effects of non-specific airborne and substrate-borne vibrations on aphids. J Pest Sci 95(2):949–958. https://doi.org/10.1007/s10340-021-01425-9
Pekas A, Mazzoni V, Appel H, Cocroft R, Dicke M (2023) Plant protection and biotremology: fundamental and applied aspects. Trends in Plant Sci. https://doi.org/10.1016/j.tplants.2023.06.021
Polajnar J, Eriksson A, Lucchi A, Anfora G, Virant-Doberlet M, Mazzoni V (2015) Manipulating behaviour with substrate-borne vibrations–potential for insect pest control. Pest Manag Sci 71(1):15–23. https://doi.org/10.1002/ps.3848
Powell G (2005) Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J Gen Virol 86(2):469–472
Prado E, Tjallingii WF (1994) Aphid activities during sieve element punctures. Entomol Exp Appl 72(2):157–165. https://doi.org/10.1111/j.1570-7458.1994.tb01813.x
R Core Team, R., & R Core Team. (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020
Ramaswamy K, Cocroft RB (2009) Collective signals in treehopper broods provide predator localization cues to the defending mother. Anim Behav 78(3):697–704. https://doi.org/10.1016/j.anbehav.2009.06.017
Commission Implementing Regulation No 485/2013 of 24 May 2013 amending implementing regulation No 540/2011, as regard the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds with plant protection products containing those active substances OJ L, 139 (2013), pp. 12–26, 25.5.2013
Commission Implementing Regulation (EU) 2018/113 of 24 January 2018 renewing the approval of the active substance acetamiprid in accordance with regulation (EC) no 1107/2009 of the European Parliament and of the council concerning the placing of plant protection products on the market, and amending the annex to commission implementing regulation (EU) No 540/2011.
Ricupero M, Desneux N, Zappalà L, Biondi A (2020) Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 247:125728. https://doi.org/10.1016/j.chemosphere.2019.125728
Roitberg BD, Myers JH (1978) Adaptation of alarm pheromone responses of the pea aphid Acyrthosiphon pisum (Harris). Can J Zool 56(1):103–108. https://doi.org/10.1139/z78-014
Sánchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N (2016) Are bee diseases linked to pesticides?—A brief review. Environ Int 89:7–11. https://doi.org/10.1016/j.envint.2016.01.009
Sarria E, Cid M, Garzo E, Fereres A (2009) Excel Workbook for automatic parameter calculation of EPG data. Comput Electron Agric 67(1–2):35–42. https://doi.org/10.1016/j.compag.2009.02.006
Seok JG, Kang TJ, Kim YG (2010) Sound stress induces developmental alterations and enhances insecticide susceptibility in the green peach aphid, Myzus persicae. Korean J Pestic Sci 14(4):415–420
Serrão JE, Plata-Rueda A, Martínez LC, Zanuncio JC (2022) Side-effects of pesticides on non-target insects in agriculture: a mini-review. Sci Nat 109(2):17. https://doi.org/10.1007/s00114-022-01788-8
Shahid E, Khan DJ, Qaisrani M, Noman M, Rani A, Ali S (2021) Effect of pesticide residues on agriculture crops. J Toxicol Pharmaceut Sci 5:18–23
Sharma A, Kumar V, Thukral AK, Bhardwaj R (2019) Responses of plants to pesticide toxicity: an overview. Planta Daninha 37:e019184291. https://doi.org/10.1590/S0100-83582019370100065
Strauß J, Stritih-Peljhan N, Nieri R, Virant-Doberlet M, Mazzoni V (2021) Communication by substrate-borne mechanical waves in insects: From basic to applied biotremology. Advances in insect physiology, vol 61. Academic Press, London, pp 189–307. https://doi.org/10.1016/bs.aiip.2021.08.002
Tholt G, Kis A, Medzihradszky A, Szita É, Tóth Z, Havelda Z, Samu F (2018) Could vectors’ fear of predators reduce the spread of plant diseases? Sci Rep 8(1):8705. https://doi.org/10.1038/s41598-018-27103-y
Thompson DA, Lehmler HJ, Kolpin DW, Hladik ML, Vargo JD, Schilling KE, Field RW (2020) A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ Sci: Process & Impacts 22(6):1315–1346. https://doi.org/10.1039/C9EM00586B
Tjallingii WF (1978) Electronic recording of penetration behaviour by aphids. Entomol Exp Appl 24(3):721–730. https://doi.org/10.1111/j.1570-7458.1978.tb02836.x
Tjallingii WF (2006) Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57(4):739–745. https://doi.org/10.1093/jxb/erj088
Troczka BJ, Singh KS, Zimmer CT, Vontas J, Nauen R, Hayward A, Bass C (2021) Molecular innovations underlying resistance to nicotine and neonicotinoids in the aphid Myzus persicae. Pest Manag Sci 77(12):5311–5320. https://doi.org/10.1002/ps.6558
Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ (2012) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol 42(3):155–163. https://doi.org/10.1016/j.ibmb.2011.11.008
Virant-Doberlet M, Kuhelj A, Polajnar J, Šturm R (2019) Predator-prey interactions and eavesdropping in vibrational communication networks. Front Ecol Evol 7:203. https://doi.org/10.3389/fevo.2019.00203
Yack J (2016) Vibrational signaling. Insect Hear. https://doi.org/10.1007/978-3-319-28890-1_5
Zapponi L, Nieri R, Zaffaroni-Caorsi V, Pugno NM, Mazzoni V (2023) Vibrational calling signals improve the efficacy of pheromone traps to capture the brown marmorated stink bug. J Pest Sci 96(2):587–597. https://doi.org/10.1007/s10340-022-01533-0
Acknowledgements
We thank Prof. Mauro Mandrioli (University of Modena and Reggio Emilia) for providing the Myzus persicae colony and Bioplanet Italia for Aphidius colemani and Adalia bipunctata. The study was partly supported by the European Union Horizon 2020 Research and Innovation project, under grant agreement no. 773431 RELACS (Replacement of Contentious Inputs in Organic Farming Systems), by the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022, Agritech National Research Center), and by the European Union FSE-REACT-EU, PON Research and Innovation 2014-2020 DM1062 / 2021. This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zippari, C., Nieri, R., Hamouche, Z. et al. Substrate-borne vibrations produced during the interaction with natural enemies alter aphids probing behavior. J Pest Sci (2024). https://doi.org/10.1007/s10340-024-01761-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-024-01761-6