Abstract
Fruit fly ground-dwelling stages (late third instar larvae, pupae, and teneral adults) are susceptible to predation from generalist ground-dwelling predators and to infection by entomopathogenic fungi (EPF). The effect of predators can be enhanced with cover crops and that of EPF by augmentative releases. However, whether these two biological control methods could be combined has not been studied under field conditions yet. Here, we studied in the field whether the enhanced activity of predators against the medfly, Ceratitis capitata, already observed in a Lolium arundinaceum ground cover could be impaired by a soil application of Metarhizium brunneum. Our results show that C. capitata adult emergence was reduced by this EPF for up to three months after fungal application, with the combination of the cover and M. brunneum being the most effective at reducing C. capitata emergence relative to bare soil (92.5% reduction). Although M. brunneum reduced the activity density of ground-dwelling predatory beetles up to 93 days after application, it showed no clear negative effects on earwigs, no effects on spiders, and a positive effect on ants up to 65 days after application. Therefore, the combined use of a ground cover of L. arundinaceum and M. brunneum against the soil-dwelling stages of C. capitata seems to work synergistically and appears as a strong and sustainable control tactic against the medfly and other fruit orchard pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Medfly ground-dwelling stages are susceptible to ground predators and entomopathogenic fungi
-
Ground covers are used as conservation biological control to enhance the impact of these predators
-
Augmentative releases of M. brunneum can be used to control C. capitata
-
A cover of tall fescue and M. brunneum was the most effective combination against C. capitata
-
Activity density of ground beetles but not other predators was temporarily reduced by M. brunneum
Introduction
The family Tephritidae is the most species-rich family within Diptera, with more than 5000 described species (EFSA PLH Panel 2020). Among these species, around 35% have been documented to attack fruit and are thus considered as true fruit flies (EFSA PLH Panel 2020). The economic importance of these flies is recognized as major worldwide (De Meyer and Ekesi 2016). The Mediterranean fruit fly, Ceratitis capitata (Wiedemann), is a highly invasive agricultural pest, endemic to most sub-Saharan countries and reported on more than 300 plant species including fruits, vegetables, and nuts (CABI 2023). Fruit rotting following oviposition and the consequent larval feeding on mature fruits may result in complete yield loss (Weldon 2020). In temperate areas, most severe damage occurs in stone fruit and citrus, where this species finds a refuge during winter (Jacas et al. 2010). Economic costs include direct crop losses, control and prevention of C. capitata infestation (both pre- and post-harvest), and limited or loss of access-free export markets (Cruz-Miralles et al. 2022). Total damage caused by tephritids in crop production, harvesting, packing, and marketing activities worldwide was estimated to amount to more than two billion dollars annually (Enkerlin et al. 2017).
Until recently, the preferred control option for fruit flies in general and especially for C. capitata was based on the use of insecticides, often in combination with food attractants targeting adult flies (Dias et al. 2022, CABI 2023). However, negative effects on biodiversity and human health caused by pesticides have prompted National Plant Protection Organizations to progressively implement policies aimed at reducing pesticide use (Mathis et al. 2022). This situation has increased the interest in alternative more sustainable methods to control C. capitata, including fruit bagging, sanitation, mass trapping, biological control, or the sterile insect technique, often combined in the context of area-wide control programs (Pla et al. 2021; Cruz-Miralles et al. 2022). Among these methods, biological control is becoming increasingly important. In a recent review of the biological control of fruit flies, Dias et al. (2022) showed that the most studied species was indeed C. capitata. These authors also found that 22% (n = 50) of the studies considered corresponded to natural/conservation biological control, whereas classical and augmentative biological control accounted for 6% (n = 13) and 5% (n = 11) of these studies, respectively. Parasitoids were the most studied fruit fly biological control agent, followed by entomopathogenic fungi (EPF), nematodes (EPN), bacteria, and predators. The fruit fly stage targeted by parasitoids is mostly the larva within the fruit, whereas those targeted by EPF, EPN, and predators are mostly those occurring in the soil, namely late third instar larvae (LIII, which drop from the fruit to the ground, burrow in the soil and pupate), pupae, and teneral adults, which remain on the ground until they can fly. These stages are susceptible to predation by generalist ground-dwelling predators (Monzó et al. 2008, 2011) and to infection by EPF (Quesada-Moraga et al. 2006; Garrido-Jurado et al. 2011a; c) and EPN (Kapranas et al. 2023). The effect of EPF and EPN can be increased by periodical applications of formulated microbials (augmentative biological control) (Scheepmaker and Butt 2010; Köhl et al. 2019), while that of predators, which include beetles, ants, earwigs, and spiders, can be enhanced through habitat manipulation (conservation biological control) (Cruz-Miralles et al. 2022). Whether these two biological control methods could be combined has not been studied yet.
A recent study investigated the association between three ground cover management options (bare soil, a seeded cover of Lolium arundinaceum (= Festuca arundinacea), and a mulch of straw), the emergence success of C. capitata, and the activity density of the most important groups of ground-dwelling predators (Cruz-Miralles et al. 2022). Reduced C. capitata emergence in the seeded cover of L. arundinaceum relative to bare soil (29% reduction) was related to higher diversity and activity density of ground-dwelling predators in the seeded cover, where more complex relationships among predators and between them and medfly occurred relative to the other options. Three predatory groups (beetles, ants, and earwigs) were negatively related to C. capitata emergence in that cover. Although the activity density of the most abundant predator group in that study, spiders, was not negatively related to C. capitata emergence, their important role should not be ruled out. That result could be attributed to spiders feeding mostly on either LIII before burial or teneral adult flies but not on buried pupae, two stages which were not properly considered in that study. Indeed, Monzó et al. (2008) proved that Pardosa cribata Simon (Araneae, Lycosidae), the most abundant ground-dwelling spider in citrus orchards in eastern Spain, could prey on both LIII and adults but not on pupae, with a preference for adult fruit flies.
The virulence of EPF toward other tephritids such as the olive fruit fly Bactrocera oleae (Rossi) adults and preimaginals has been demonstrated both at laboratory and field conditions, with even an existing commercial development of a control strategy based on the ground application of Metarhizium brunneum Petch. (Ascomycota: Hypocreales: Clavicipitaceae) beneath the tree canopy targeting preimaginals (Yousef et al. 2017; 2018). However, there is a lack of such developments for other key tephritids such as C. capitata. The pathogenicity of EPF has been widely evaluated for preimaginal C. capitata control in laboratory conditions during the last 20 years (Ekesi et al. 2002; Quesada-Moraga et al. 2006; Beris et al. 2013), particularly several aspects related to the influence of (1) abiotic factors in the infection ability (Ekesi et al. 2003; Garrido-Jurado et al. 2011a, c; Gava et al. 2021), (2) natural enemies (Gava and Paranhos 2023), (3) associated endoparasitoids (Ekesi et al. 2005), and (4) pesticides (Mochi et al. 2006; Yousef et al. 2015). However, field or semifield experiments evaluating any of these interactions together or individually have not been undertaken yet (Gava et al. 2021). Moreover, the use of a ground cover in combination with EPF has not been dealt with yet. Considering that the adoption of cover crops is a key agricultural tactic to achieve the EU’s ambitions to reduce EU greenhouse gas emissions by 2030 (Riviere et al. 2022), the evaluation of the compatibility of any pest control measure targeting the soil with natural or artificial ground covers is urgently needed. As to date, we are not aware of any study addressing the compatibility of ground cover management and the application of EPF to control C. capitata; in this study, we have investigated the effect of bare soil and a seeded cover of L. arundinaceum when either treated or not with M. brunneum on the emergence of C. capitata. To ascertain any side-effect of M. brunneum on the most relevant groups of ground-dwelling predators, we have also evaluated the effect of these treatment combinations on the activity density of the main groups of generalist ground-dwelling predators (spiders, beetles, ants, and earwigs). Our objective has been to explore the association between these treatment combinations and (1) the emergence success of C. capitata; and (2) the abundance of the most important groups of ground-dwelling predators of C. capitata. We challenged the hypothesis that the enhanced activity of predators in the seeded cover of L. arundinaceum could be impaired by the application of M. brunneum, which could therefore be incompatible.
Materials and methods
Experimental orchard
Experiments were carried out from June to December 2022 in a 1 ha citrus orchard located in Les Alqueries (Spain, 39° 54′ N; 00° 06′ W), already used in previous studies on the effects of ground cover management on the emergence of C. capitata (Cruz-Miralles et al. 2022). Trees were 20-year-old “Clemenules” mandarins [Citrus clementina Tanaka (Rutaceae)] grafted on citrange Carrizo (Poncirus trifoliata (L.) Rafinesque × Citrus sinensis (L.) Osbeck). Rows were 6 m apart and followed a N-S orientation. Within rows, trees were spaced 4 m, drip-irrigated, and maintained weed-free by herbicide treatments (glyphosate). Twenty-four adjacent trees in two consecutive rows (12 trees per row) in the middle of the orchard were removed in 2014 and replanted in late summer 2019 with 2-year-old clementine trees (same scion-rootstock combination as before). These 24 trees were individually enclosed in an aluminum cage (4 × 4 × 4 m) covered with a rigid nylon mesh (10 × 14 threads cm−2) on all sides but one (the western side of the western row and the eastern side of the eastern row). These cages received one of the two ground management treatments considered in this study, which were randomly distributed within each row (i.e., 6 cages per treatment and row; see Fig. 1 Supplementary). The first treatment was bare soil by means of herbicide application (glyphosate + MCPA). The second treatment consisted of a homogeneous cover of L. arundinaceum, which was established in early autumn 2019 and subsequently mowed twice per season with grass clippings left in place. In this treatment, the vicinity of the tree was maintained free of L. arundinaceum by mechanical means. A datalogger (CEM, model DT-171; www.cem-instruments.com/en/Product/detail/id/980), which continuously monitored temperature and relative humidity values at 1-h intervals, was set in one of the cages. Daily rainfall measurements were obtained from the meteorological station of Vila-Real (http://riegos.ivia.es/listado-de-estaciones/vila-real), which is located 5 km NW of the orchard.
Fungal strain, cultivation, and inoculum production
The EAMa 01/58-Su M. brunneum strain used in this study was obtained from the culture collection at the Agronomy Department of the University of Cordoba. This strain was originally isolated from soil in a wheat plantation at Hinojosa del Duque, Córdoba, Spain. The strain was deposited in the Spanish collection of culture types (CECT) with accession number CECT 20764. This fungal strain was licensed to Koppert B.V., Netherlands, in 2021. The cultivation and inoculum production for the experiment were performed as described by Yousef et al. (2017).
Soil application of the fungal suspension
For each ground management option (bare soil, BS, and a seeded cover of L. arundinaceum, LA), cages were randomly assigned to one of the following three groups: control, one, and two applications of the fungal suspension (C, A1, and A2 in Fig. 1 Suppl., respectively) with four replicates (two per row) for each treatment * application combination. On June 28, 2022, the soil of the cages assigned to A1 and A2 was sprayed with 1 l of a suspension of M. brunneum EAMa 01/58-Su strain, which contained 2.5 g of conidia or 1 × 108 viable conidia, using an atomizer (Yousef et al. 2017). In the control cages, the soil was sprayed with 1 l of water. In all the experiments and subsequent to fungal application, the presence of M. brunneum in the soil was monitored. For this, soil samples were collected immediately after the treatment and then, once per month, with six completely randomized soil samples collected using a soil corer (5 cm in diameter) to a depth of 15 cm. These samples were transferred to the laboratory to determine the presence of entomopathogenic fungi in the soil using a selective growing medium. Briefly, 1 g of the homogenized soil sample was added to 9 ml of sterile distilled water, and shaken for 60 min. After homogenization, aliquots of 100 μl were spread onto Petri plates containing Sabouraud glucose agar chloramphenicol medium (0.5 g/l). The plates were incubated at 25 °C for 7–10 days and colony forming units were counted (Garrido-Jurado et al. 2011a). Based on this monitoring and on results of adult emergence (see below), a second treatment was forecasted for half of the treated cages (A2 in Fig. 1 Suppl.) toward the end of summer–early autumn. At that time heavy rains, which could wash the inoculum from the soil, usually take place in the study area.
Ceratitis capitata stock colony
Third instar larvae (LIII) of the Vienna-8D53− genetic sexing strain obtained from a mass-rearing facility located in Caudete de las Fuentes (Valencia, Spain) were used in our assays. These insects were reared on an artificial diet at a temperature of 25.8 ± 0.3 °C and 91.5 ± 1.5% RH at dark (Pla et al. 2021).
C. capitata management
From June to November 2022, once or twice per month (depending on results), around 3000 mature third instar larvae (LIII) were refrigerated (9 °C) and transferred to UJI facilities, where they were split in groups of 100. Two of these groups were kept in the laboratory and 24 were further processed in the field. One of the two laboratory groups was introduced into a 10 cm in diameter Petri dish, which was wrapped in aluminum foil to keep it dark and transferred to a climatic cabinet set at 25:20 °C and a 12:12 L:D photoperiod. The other laboratory group was set on top of a 21.5 cm diameter and 19 cm high pot containing a mixture of vermiculite and peat (1:3; vol:vol) watered to field capacity and similarly transferred to the same climatic cabinet. Pupation, adult emergence, and sex ratio were scored. The former two values were used as control to correct values recorded for field-released insects (see below) while the sex ratio was used as reference to detect any difference with field-released insects (see below).
The 24 groups of 100 LIII each were eventually released in the field, one per cage. Larvae were released in an emergence trap (see Cruz-Miralles et al. 2022 for details) consisting of a 15-cm long and 12 cm diameter toilet soil pipe fitted to a bottomless transparent PVC bottle of the same diameter and 35 cm long, with a zenithal ventilation hole covered with a mesh to prevent water condensation. The pipe and the bottle were fitted with a rubber band. Prior to the release, a hole of the same dimensions as the soil pipe was dug by carefully extracting a soil cylinder. Then, the pipe was put in place and the previously removed soil cylinder introduced into the pipe. The 100 LIII were distributed on the surface of the trap, which was covered with the bottomless bottle to protect them from predation. Two days later the bottles were removed and the number of individuals (either larvae or pupae) remaining on the top of the trap were counted and removed. This figure was considered as representative of unsuccessful larval burial (i.e., individuals either dead or fully exposed to predation). Then, the trap was left uncovered to allow full exposure of the buried individuals to natural enemies and abiotic stressors (i.e., rainfall). Based on the temperatures measured in the orchard, the thermal constant and the lower development threshold of C. capitata (260 DD and 11 °C, respectively; Escudero-Colomar et al. 2008), the emergence date was estimated. One week before reaching that date, the bottomless bottle was set again in place. At that moment, the bottle included a transparent plastic sheet sprayed with tangle-trap® covering the inner wall of the bottle. Traps were periodically inspected and adults found stuck on the glue were counted, removed, and taken to the laboratory for further processing (see below). The whole trap was removed after seven days with no adult captures. Field unsuccessful larval burial (% of total LIII) and adult emergence (% expressed on both total LIII and those successfully buried) were calculated based on the number of larvae and adults counted. These percentages were corrected with the corresponding laboratory values (Abbott 1925). As soon as the traps were removed (from 15 to 30 days after initial setup), a new set of larvae and traps was put in place.
Specimens stuck on the glue of the emergence trap were taken to the laboratory to ascertain whether they were infected by M. brunneum. These flies were immediately surface-sterilized with 1% sodium hypochlorite followed by three rinses with sterile distilled water for 1 min each. They were then placed on sterile wet filter paper in sterile petri plates, sealed with parafilm, and kept at 25 °C to be inspected for fungal outgrowth on the cadavers (Quesada-Moraga et al. 2006).
Pitfall traps
From June to December 2022, to identify potential ground predators of C. capitata, a pitfall trap was set in each cage (24 traps in total). Traps consisted of a plastic jar (12.5 cm diam., 12 cm depth), with a plastic funnel fitted to its upper edge. A plastic container (250 ml) half filled with a 3:1 mixture of water and ethanol, and 1.25% antifreeze, was placed inside the plastic cup. Traps were serviced every 15 days. Captures were transported to the laboratory, where they were sorted out. Specimens were determined to family level under a binocular microscope and categorized into the following generalist soil-dwelling predator groups: Coleoptera (Carabidae and Staphylinidae), Dermaptera (Forficulidae), Formicidae, and Araneae (Lycosidae).
Statistical analyses
Corrected unsuccessful larval burial (%), adult emergence (%; relative to the initial 100 LIII and to those LIII successfully buried in the soil), sex ratio (females/total), adult infection (%), and predator counts for Coleoptera, Formicidae, Dermaptera, and Araneae (activity density; number per trap per day) were modeled using generalized linear mixed models (GLMM) with a negative binomial distribution of the error and a logit link function, with treatment (M. brunneum or control), cover (BS or LA), and date as fixed factors and replicate (= cage) as a random factor. All interactions (2- and 3-factor) were considered. Once differences along time were established, for each date, (1) unsuccessful larval burial and adult emergence were further modeled using general linear models (GLM) with a binomial distribution of the error and logit link function, and (2) predator counts were modeled using GLM with a quasi-Poisson distribution of the error and a logit link function. In all cases, treatment, cover, and their interaction were considered as fixed factors. Models were refined by progressively removing non-significant (P > 0.05) factors. Akaike information criterion (AIC) (Akaike 1974) was used to select the best model. When necessary, pairwise comparisons were made using Tukey post hoc test (P < 0.05). The R software (R Core Team 2023) was used to fit the models. The package lme4 (Bates et al. 2015) was used to fit both GLMMs and GLMs, and the package multcomp (Hothorn et al. 2008) was used to perform the pairwise comparisons.
Results
Mean monthly values of temperature and relative humidity ranged from 13.3 to 27.6 °C (mean: 21.9 °C) and 55 to 87.3% (mean: 75.2%), respectively, during the study period. The absolute minimum and maximum temperatures ranged from 5.8 to 20.3 °C and 24.9 to 40.3 °C, respectively. A total of 321.5 mm of rain were registered during this period, with 39.6 mm on June 27, the day prior to the application of the fungus, and two episodes of heavy rain (> 40 mm in 24 h) on November 11 and 12. From the application day to October 5, rain occurred only 8 times for a total of 18.7 mm during this 100-d period (Fig. 1).
Fungal inoculum was found in treated cages only and no Metarhizium species were found in the untreated cages. The concentration of M. brunneum EAMa 01/58-Su strain ranged from 5.0·104 to 8.3·105 CFU g−1 soil for bare soil, 147 and 14 days after treatment, respectively (Fig. 1). As a consequence, no additional fungal application was made at the end of the summer as initially planned, which eventually made the number of replicates (cages) for the fungus-treated cages double than control (8 versus 4 replicates per cover for fungus-treated and control cages, respectively). Although sentinel LIII were introduced into the cages at monthly intervals, no adults could be recovered for those released beyond day 87 after the fungal application (September 29, 2022). Therefore, the experiment was discontinued in December 2022.
Larval successful pupation in the laboratory was 100%. Consequently, no correction was needed for field-collected data. Successful larval burial into the soil (Fig. 2) was significantly affected by time since fungal application (z-value = − 13.22; P < 0.001) and its interaction with fungal application (z-value = − 5.03; P < 0.001) and cover (z-value = 13.72; P < 0.001). All other interactions were not significant (AIC = 2809.3 and df = 137 for the model). When each date was separately analyzed (Table 1), with the exception of the first date after application, successful burial was significantly higher in bare soil relative to L. arundinaceum with no clear effect of the fungal application (Table 1).
Adult emergence in the laboratory ranged from 90.5 to 94.5%. These values were used to correct field-collected data (Fig. 2). Field adult emergence (irrespective of whether referred to the 100 sentinel LIII released or to those successfully buried into the soil) was affected by fungal application (z-value = − 2.16; P = 0.030 and z-value = − 1.91; P = 0.050, respectively), time since application (z-value = 10.18; P < 0.001 and z-value = 8.39; P < 0.001, respectively) and the interactions of time with fungal application (z-value = − 2.86; P = 0.004 and z-value = − 3.11; P = 0.002, respectively) and cover (z-value = − 3.73; P < 0.001 and z-value = − 3.14; P = 0.002, respectively). All other interactions were not significant (AIC = 652.8 and 629.2, respectively, and df = 89 in both models). When each date was separately analyzed (Tables 2 and 3), with the exception of the first date after application, adult emergence was higher in bare soil relative to L. arundinaceum and in control relative to M. brunneum-treated cages (Tables 2 and 3). As consequence, the efficacy of the different combinations tested relative to bare soil at inhibiting adult emergence ranged from 59.4 to 92.5% in fungus-treated bare soil and fungus-treated L. arundinaceum, respectively. The sex ratio of these adults ranged from 0.06 to 0.58 females/total, with differences between dates (AIC = 246.7; z-value = 2.215; df = 71; P = 0.026) but no significant effects of the fungal treatment, the cover, or their interactions. Adult infection ranged from 5.1% for fungus-treated bare soil 87 days after the treatment to 67.3% for fungus-treated L. arundinaceum 64 days after the treatment. However, the low number of adults emerged and recovered from fungus-treated cages (Table 2) precluded the successful modeling of these results.
The results of the GLMM applied to the activity density of the different groups of ground-dwelling predators are shown in Table 4. Time since fungal application was significant in all cases, the fungal application was significant for all groups except for spiders, the cover was also significant in all groups except for ants, and the only significant interaction, treatment*time, occurred in earwigs. When each group was separately analyzed by date, for beetles, treatment was significant (P < 0.05) only 65 and 93 days after treatment, whereas cover was not significant (P > 0.05) 7, 21, 65, and 135 days after treatment. When significant, beetle captures were higher in L. arundinaceum relative to bare soil and in those two dates where the treatment was also significant, higher values were observed in control cages (Fig. 3). On average, staphylinids and carabids represented around 50% of total captures each in all four treatment combinations. For ants, treatment was significant only 36 and 65 days after treatment. On both dates, ant captures were lower in control cages (Fig. 4). For earwigs neither treatment nor cover or their interaction were ever significant (Fig. 5). Finally, for spiders (mostly Lycosidae), cover was significant for all dates up to 107 days after treatment, with higher spider captures in L. arundinaceum compared to bare soil. Afterward, there were no differences between covers (Fig. 6).
Coleoptera activity density in 4 × 4 × 4 m3 cages with either bare soil (BS) or a seeded cover of L. arundinaceum (LA) combined with a treatment of M. brunneum (T; applied on June 28, 2022) or control (C). Pitfall traps were sampled every 15 days from treatment to 11 December, 2022; Dots and triangles represent the measured values as lines, the GLMMs fitted. Lines are significantly different (P < 0.050, see Table 4)
Formicidae activity density in 4 × 4 × 4 m3 cages with either bare soil (BS) or a seeded cover of L. arundinaceum (LA) combined with a treatment of M. brunneum (T; applied on June 28, 2022) or control (C). Pitfall traps were sampled every 15 days from treatment to 11 December, 2022; Dots and triangles represent the measured values as lines, the GLMMs fitted. Lines are significantly different (P < 0.050, see Table 4)
Dermaptera activity density in 4 × 4 × 4 m3 cages with either bare soil (BS) or a seeded cover of L. arundinaceum (LA) combined with a treatment of M. brunneum (T; applied on June 28, 2022) or control (C). Pitfall traps were sampled every 15 days from treatment to 11 December, 2022; Dots and triangles represent the measured values as lines, the GLMMs fitted. Lines are significantly different (P < 0.050, see Table 4)
Araneae activity density in 4 × 4 × 4 m3 cages with either bare soil (BS) or a seeded cover of L. arundinaceum (LA) combined with a treatment of M. brunneum (T; applied on June 28, 2022) or control (C). Pitfall traps were sampled every 15 days from treatment to 11 December, 2022; Dots and triangles represent the measured values as lines, the GLMMs fitted. Lines are significantly different (P < 0.050, see Table 4)
Discussion
In agreement with previous results (Cruz-Miralles et al. 2022), the L. arundinaceum cover alone proved effective at reducing C. capitata larval burial and adult emergence compared to bare soil (14.4 and 48.9% mean reductions, respectively). Due to the short time elapsed between the fungal application and the evaluation of larval burial (2 days), as expected, no effect of M. brunneum on burial success was detected. However, adult emergence was reduced by M. brunneum for up to three months after fungal application, with the combination of L. arundinaceum and M. brunneum being the most effective at reducing C. capitata emergence relative to bare soil (92.5%; Fig. 2). Although M. brunneum also reduced the activity density of ground-dwelling predatory beetles (up to 93 days after the application), it showed no clear negative effects on earwigs, no effects on spiders, and a positive effect on ants (up to 65 days after the application), as already observed in olive groves for the same strain of M. brunneum (Garrido-Jurado et al. 2011b). Therefore, our initial hypothesis that the enhanced activity of predators in the seeded cover of L. arundinaceum could be impaired by M. brunneum, proved only partially correct. Yet, the combined use of a ground cover and M. brunneum against the soil-dwelling stages of C. capitata is compatible and they seem to work synergistically.
The lack of heavy rain from the start of the assay until mid-autumn (Fig. 2) could explain the long-term effect of M. brunneum on C. capitata observed in both bare soil and the L. arundinaceum cover. Indeed, the inoculum could be still detected in the soil 150 days after treatment at a concentration of 5 × 104 CFU g−1. Surprisingly, almost 40 and 200 mm of rain, which could have washed the fungus from the upper layers of the soil where C. capitata pupates, were registered around mid-October and mid-November, respectively. This higher-than-expected persistence of M. brunneum (keep in mind that a second fungal application toward the end of the summer had been initially planned) could be an important asset of M. brunneum. Indeed, field experiments have shown that this fungus can persist over 250 days in the soil (Hernández et al. 2023). This result, though, could also be related to M. brunneum multiplying in the soil when insect hosts are present, as shown in studies where fungal concentration increased in the presence of susceptible hosts (Vestergaard et al. 2003; Meyling and Eilenberg 2007; EFSA 2020). The infected teneral adults of C. capitata recovered in our study can be taken as evidence of the reproduction of M. brunneum EAMa 01/58-Su strain in the soil. Interestingly, our methodology, which included the washing of the adults captured in the traps before their processing, likely underestimated the impact of M. brunneum on emerged flies, as the percentage of infested insects should most probably be higher than that of infected ones. Infection of non-target insects with M. brunneum following its soil application has been documented for Coleoptera in Danish alfalfa fields (Vestergaard et al. 2003) and Coleoptera and Hemiptera in a Danish fir plantation (Nielsen et al. 2006). Therefore, fungal infection of non-target insects could explain the lower activity density of predatory beetles recorded in fungus-treated compared to control plots (Table 4; Fig. 3). A completely different situation occurred to the other three groups of predators examined in this study (ants, earwigs, and spiders). As soil-dwelling arthropods have co-evolved with pathogenic microorganisms, which are widespread in the soil, like EPF, these arthropods have developed strategies to cope with infection (Vanninen et al. 1999). In turn, these strategies have been overcome by entomopathogenic microorganisms in an ongoing arms race (Ortiz-Urquiza and Keyhani 2013). Tephritids, for instance, present a puparium, which makes the pupal stage less susceptible to EPF (Ekesi et al. 2002). This has been related to the nature of the puparium, which originates from the cuticle of LIII, which remains in tephritids as an extra protecting layer for the pupa conferring a barrier to penetration by EPF (Ekesi et al. 2002). Indeed, the cuticle is the first and major protective barrier against pathogen infections in arthropods. Puparia, though, do not occur in ants, which did even better in M. brunneum-treated than in control plots, in spiders, for which this fungus was neutral, and earwigs. Ants, produce antimicrobial secretions inhibiting EPF (Bot et al. 2001; Rodrigues et al. 2009; Garrido-Jurado et al. 2011b), and can avoid and differentially manage prey depending on their infectious potential (Pereira and Detrain 2020), a phenomenon observed in larvae of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) as well (Ríos-Moreno et al. 2018). Additionally, ants, as other eusocial insects, have evolved many effective defense mechanisms against pathogens (Cremer et al. 2007; Meunier 2015), like self- and allo-grooming (Reber et al. 2011) or nest cleaning (Bot et al. 2001; Jackson and Hart 2009; Diez et al. 2012). These behaviors, which complement individual immune defense are called social immunity (Diehl and Meunier 2018). Because Cruz-Miralles et al. (2022) identified the occurrence of intra-guild interference (which could include competition, predation, or risk avoidance behavior) between ants and beetles in a cover of L. arundinaceum, the effective defense mechanisms identified in ants may have allowed this group to have an additional advantage over ground-dwelling beetles resulting in an ant-mediated negative effect of M. brunneum on beetles. Hence, the negative effects observed on beetles in our study (Fig. 3) could be attributed to both the direct and indirect effects of fungal application.
Social immunity is not exclusive to eusocial insect species, like ants, bees, or termites, and can be found in less complex forms of group living, such as the simple family units of earwigs. In these units, parents may cover the nest with feces with antimicrobial activity (Diehl et al. 2015) and females, which do not avoid EPF-contaminated environments, can mitigate the associated costs of pathogen exposure by adjusting their level of egg care (Diehl and Meunier 2018). In particular, the presence of pathogens shortened the duration of egg abandonment, which favors the removal of pathogens from the egg surface (Boos et al. 2014) and favored the construction of a nest, a form of hygienic behavior that may help cleaning the nest from pathogen spores by shifting the sands around (Arathi et al. 1999). Same as with ants, social immunity in earwigs could explain the lack of clear negative impacts of the application of M. brunneum on the activity density of this group of omnivorous predators (Fig. 5). Although Fischhoff et al. (2018) reported reduced survival of the wolf spider Schizocosa ocreata (Hentz) (Araneae: Lycosidae) after soil application of M. brunneum in a field microcosm study, these authors suggested that potential interference between fungal application and S. ocreata should be examined at larger scales. Our study provides evidence of absence of impact of M. brunneum EAMa 01/58-Su strain on ground-dwelling spiders (Fig. 6). To sum up, these results point at this strain as mostly safe for the predatory guild of C. capitata occurring in the soil. The harmful effects detected on beetles disappeared three months after the fungal application. Therefore, the simultaneous use of a ground cover of L. arundinaceum as a conservation biological control method to increase the populations of the predators of C. capitata, and that of M. brunneum as an augmentative biological control method, can enhance the control efficacy against the soil-dwelling stages of this fruit fly. Moreover, as the use of L. arundinaceum also favors the conservation along the year of some key above-ground natural enemies of other important fruit pests, like mites, thrips, and aphids (Aguilar-Fenollosa et al. 2011a, b, c; Aguilar-Fenollosa and Jacas 2013; Gómez-Marco et al. 2015; Gómez-Martínez et al. 2018), such a ground cover appears as a strong and sustainable conservation biological control method against fruit orchard pests.
Commercial fruit orchards, including citrus, usually contain one single cultivar or two, when they are self-incompatible, but usually reach harvest maturity at around the same time. As fruit flies prefer fully ripe fruit to oviposit (Aluja and Mangan 2008), most fruit within an orchard become susceptible to C. capitata around the same time. The window period for C. capitata oviposition could extend for about two months, from the moment when fruit become receptive until unharvested fruit either mummify or decompose and can no longer support C. capitata immature development (this window would shrink in case that sanitation is applied to unharvested fruit). Therefore, one single treatment of M. brunneum applied before harvest, with an efficiency extending up to three months, could be enough to reduce the emergence of flies developing on those fruits. Only in case of heavy rain, a second treatment could be advisable. Because such a treatment would not reduce damage in the orchard where the application takes place during the ongoing season but reduce the density of further developing adult populations, it could ideally be part of an area-wide IPM program, where this ground application could target hotspots on a collective basis. To calculate the cost for the farmer of the different combinations considered in this study, we have followed (1) Aguilar-Fenollosa et al. (2011c) for the establishment and maintenance of bare soil and the seeded cover of L. arundinaceum and (2) Sosa-Gómez (2017) and Lee et al. (2023) for the soil application of M. brunneum. Based on these calculations, the most cost-effective tactic would be the use of a ground cover of L. arundinaceum in combination with a single soil application of M. brunneum (Table 5).
Author contributions
JAJ and EQM conceived and designed research. JCM, YM, and IGJ conducted the experiments. OD contributed C. capitata larvae. MVI and JAJ analyzed data. JAJ prepared the first draft of the manuscript, which was read, modified, and eventually approved by all authors.
Data availability
Raw data will be available at UJI public repository (http://repositori.uji.es/xmlui/).
References
Abbott WS (1925) A Method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. https://doi.org/10.1093/jee/18.2.265a
Aguilar-Fenollosa A, Jacas JA (2013) Effect of ground cover management on Thysanoptera (thrips) in clementine mandarin orchards. J Pest Sci 86:469–481. https://doi.org/10.1007/s10340-013-0494-x
Aguilar-Fenollosa E, Ibáñez-Gual MV, Pascual-Ruiz S, Hurtado-Ruiz M, Jacas JA (2011a) Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): tetranychid mite dynamics. Biol Control. https://doi.org/10.1016/j.biocontrol.2011.06.012
Aguilar-Fenollosa E, Ibáñez-Gual MV, Pascual-Ruiz S, Hurtado M, Jacas JA (2011b) Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (II): top-down regulation mechanisms. Biol Control 59:171–179. https://doi.org/10.1016/j.biocontrol.2011.06.012
Aguilar-Fenollosa E, Pascual-Ruiz S, Hurtado MA, Jacas JA (2011c) Efficacy and economics of ground cover management as a conservation biological control strategy against Tetranychus urticae in clementine mandarin orchards. Crop Prot 30(10):1328–1333. https://doi.org/10.1016/j.cropro.2011.05.011
Akaike H (1974) A new look at the statistical model identifcation. IEEE Trans Autom Control 19:716–723
Aluja M, Mangan RL (2008) Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Annu Rev Entomol 53:473–502. https://doi.org/10.1146/annurev.ento.53.103106.093350
Arathi HS, Burns I, Spivak M (1999) Ethology of hygienic behavior in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioral repertoire of hygienic bees. Ethology 106:365–379. https://doi.org/10.1046/j.1439-0310.2000.00556.x
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Beris EI, Papachristos DP, Fytrou A, Antonatos SΑ, Kontodimas DC (2013) Pathogenicity of three entomopathogenic fungi on pupae and adults of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J Pest Sci 86:275–284. https://doi.org/10.1007/s10340-012-0468-4
Boos S, Meunier J, Pichon S, Kölliker M (2014) Maternal care provides antifungal protection to eggs in the European earwig. Behav Ecol 25:754–761. https://doi.org/10.1093/beheco/aru046
Bot ANM, Currie CR, Hart AG, Boomsma JJ (2001) Waste management in leaf-cutting ants. Ethol Ecol Evol 13:225–237. https://doi.org/10.1080/08927014.2001.9522772
CABI (Centre for Agriculture and Bioscience International) (2023) Invasive species compendium. https://doi.org/10.1079/cabicompendium.12367. Accessed April 1, 2023
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693-702. https://doi.org/10.1016/j.cub.2007.06.008
Cruz-Miralles J, Guzzo M, Ibáñez-Gual MV et al (2022) Ground-covers affect the activity density of ground-dwelling predators and their impact on the Mediterranean fruit fly, Ceratitis capitata. Biocontrol 67:583–592. https://doi.org/10.1007/s10526-022-10168-0
De Meyer M, Ekesi S (2016) Exotic invasive fruit flies (Diptera: Tephritidae): in and out of Africa. In: Ekesi S, Mohamed SA, De Meyer M (eds) Fruit fly research and development in Africa-towards a sustainable management strategy to improve horticulture. Springer, Switzerland, pp 127–150
Dias NP, Montoya P, Nava DE (2022) A 30-year systematic review reveals success in tephritid fruit fly biological control research. Entomol Exp Appl 170:370–384. https://doi.org/10.1111/eea.13157
Diehl JMC, Meunier J (2018) Surrounding pathogens shape maternal egg care but not egg production in the European earwig. Behav Ecol 29(1):128–136. https://doi.org/10.1093/beheco/arx140
Diehl JM, Körner M, Pietsch M, Meunier J (2015) Feces production as a form of social immunity in an insect with facultative maternal care. BMC Evol Biol 15:40. https://doi.org/10.1186/s12862-015-0330-4
Diez L, Deneubourg JL, Detrain C (2012) Social prophylaxis through distant corpse removal in ants. Naturwissenschaften 99:833–842. https://doi.org/10.1007/s00114-012-0965-6
EFSA (European Food Safety Authority), Anastassiadou M, Arena M, Auteri D, Brancato A, Bura L, Carrasco Cabrera L, Chaideftou E, Chiusolo A, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Sharp R, Szentes C, Terron A, Tiramani M, Vagenende B, Villamar-Bouza L (2020) Conclusions on the peer review of the pesticide risk assessment of the active substance Metarhizium brunneum BIPESCO 5/F52. EFSA J 18(10):e06274. https://doi.org/10.2903/j.efsa.2020.6274
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen-Schmutz K, Di Serio F, Gonthier P, Jacques M-A, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, NavasCortes JA, Parnell S, Potting R, Reignault PL, Thulke H-H, Van der Werf W, Vicent Civera A, Yuen J, Zappala L, Bali EM, Papadopoulos N, Papanastassiou S, Czwienczek E, MacLeod A (2020) Pest categorisation of non-EU Tephritidae. EFSA J 18(1):e05931. https://doi.org/10.2903/j.efsa.2020.5931
Ekesi S, Maniania NK, Lux SA (2002) Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci Technol 12(1):7–17. https://doi.org/10.1080/09583150120093077
Ekesi S, Maniania NK, Lux SA (2003) Effect of soil temperature and moisture on survival and infectivity of Metarhizium anisopliae to four tephritid fruit fly puparia. J Invert Pathol 83(2):157–167. https://doi.org/10.1016/S0022-2011(03)00069-7
Ekesi S, Maniania NK, Mohamed SA, Lux SA (2005) Effect of soil application of different formulations of Metarhizium anisopliae on African tephritid fruit flies and their associated endoparasitoids. Biol Control 35(1):83–91. https://doi.org/10.1016/j.biocontrol.2005.06.010
Enkerlin W, Gutiérrez Ruelas J, Pantaleón R, Soto Litera C, Villaseñor Cortés A, Zavala López JL, Orozco Dávila D, Montoya Gerardo P, Silva Villarreal L, Cotoc Roldán E, Hernández López F, Arenas Castillo A, Castellanos Domínguez D, Valle Mora A, Rendón Arana P, Cáceres Barrios C, Midgarden D, Villatoro Villatoro C, Lira Prera E, Zelaya Estradé O, Castañeda Aldana R, López Culajay J, Ramírez y Ramírez F, Liedo Fernández P, Ortíz Moreno G, Reyes Flores J, Hendrichs J (2017) The moscamed regional programme: review of a success story of area-wide sterile insect technique application. Entomol Exp Appl 164:188–203. https://doi.org/10.1111/eea.12611
Escudero-Colomar LA, Vilajeliu M, Batllori L (2008) Seasonality in the occurrence of the Mediterranean fruit fy [Ceratitis capitata (Wied.)] in the north-east of Spain. J Appl Entomol 132:714–721. https://doi.org/10.1111/j.1439-0418.2008.01372.x
Fischhoff IR, Burtis JC, Keesing F, Ostfeld RS (2018) Tritrophic interactions between a fungal pathogen, a spider predator, and the blacklegged tick. Ecol Evol 8:7824–7834. https://doi.org/10.1002/ece3.4271
Garrido-Jurado I, Torrent J, Barrón V, Corpas A, Quesada-Moraga E (2011a) Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol Control 58(3):277–285. https://doi.org/10.1016/j.biocontrol.2011.05.017
Garrido-Jurado I, Ruano F, Campos M, Quesada-Moraga E (2011b) Effects of soil treatments with entomopathogenic fungi on soil dwelling non-target arthropods at a commercial olive orchard. Biol Control 59:239–244. https://doi.org/10.1016/j.biocontrol.2011.07.001
Garrido-Jurado I, Valverde-García P, Quesada-Moraga E (2011c) Use of a multiple logistic regression model to determine the effects of soil moisture and temperature on the virulence of entomopathogenic fungi against pre-imaginal Mediterranean fruit fly Ceratitis capitata. Biol Control 59(3):366–437. https://doi.org/10.1016/j.biocontrol.2011.09.011
Gava CAT, Paranhos BAJ (2023) Combining the virulent Beauveria bassiana (Balsam) Vuillemin LCB289 and nematode strains to control pupae of Ceratitis capitata Wiedemann. Biocontrol Sci Technol 33(4):383–396. https://doi.org/10.1080/09583157.2023.2191300
Gava CAT, da Silva JC, Simões WL, Paranhos BAJ (2021) Impact of soil texture on conidia movement and residual effect of entomopathogenic fungi applied through irrigation to control fruit-fly pupae in mango orchards. Biol Control 163:104559. https://doi.org/10.1016/j.biocontrol.2021.104559
Gómez-Marco F, Tena A, Jacas JA, Urbaneja A (2015) Early arrival of predators control Aphis spiraecola (Hemiptera: Aphididae) colonies in citrus clementine. J Pest Sci 89:69–79. https://doi.org/10.1007/s10340-015-0668-9
Gómez-Martínez MA, Jaques JA, Ibáñez-Gual MV, Pina T (2018) When the ground cover brings guests: is Anaphothrips obscurus a friend or a foe for the biological control of Tetranychus urticae in clementines? J Pest Sci 91:613–623. https://doi.org/10.1007/s10340-017-0926-0
Hernández I, Sant C, Martínez R, Almazán M, Caminal M, Quero V, El-Adak M, Casanova A, Garrido-Jurado I, Yousef-Yousef M, Quesada-Moraga E, Lara JM, Fernández C (2023) Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the soil is affected by formulation type as shown by strain-specific DNA markers. J Fungi 9(2):229. https://doi.org/10.3390/jof9020229
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Jacas JA, Karamaouna F, Vercher R, Zappalà L (2010) Citrus pest management in the northern Mediterranean basin (Spain, Italy and Greece). In: Ciancio A, Mukerji K (eds) Integrated management of arthropod pests and insect borne diseases. Integrated management of plant pests and diseases. Springer, Dordrecht, pp 3–27
Jackson DE, Hart AG (2009) Does sanitation facilitate sociality? Anim Behav 77:e1–e5. https://doi.org/10.1016/j.anbehav.2008.09.013
Kapranas A, Chronopoulou A, Peters A, Antonatos S, Lytra I, Milonas P, Papachristos D (2023) Early and off-season biological control of medfly with entomopathogenic nematodes: from laboratory experiments to successful field trials. Biol Control 179:105173. https://doi.org/10.1016/j.biocontrol.2023.105173
Köhl J, Booij K, Kolnaar R, Ravensberg WJ (2019) Ecological arguments to reconsider data requirements regarding the environmental fate of microbial biocontrol agents in the registration procedure in the European Union. Biocontrol 64:469–487. https://doi.org/10.1007/s10526-019-09964-y
Lee D, Johnson MA, Aristizábal LF, Shriner S, Chan C, Miyasaka S, Wall M (2023) Economic benefits from managing coffee berry borer (Hypothenemus hampei) in Hawaii. InSects 14(4):350. https://doi.org/10.3390/insects14040350
Mathis M, Blom JF, Nemecek T, Bravin E, Jeanneret P, Daniel O, de Baan L (2022) Comparison of exemplary crop protection strategies in Swiss apple production: multicriteria assessment of pesticide use, ecotoxicological risks, environmental and economic impacts. Sustain Prod Consum 31:512–528. https://doi.org/10.1016/j.spc.2022.03.008
Meunier J (2015) Social immunity and the evolution of group living in insects. Philos Trans R Soc B Biol Sci 370:20140102. https://doi.org/10.1098/rstb.2014.0102
Meyling N, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol Control 43:145–155. https://doi.org/10.1016/j.biocontrol.2007.07.007
Mochi DA, Monteiro AC, De Bortoli SA, Dória HO, Barbosa JC (2006) Pathogenicity of Metarhizium anisopliae for Ceratitis capitata (Wied.)(Diptera: Tephritidae) in soil with different pesticides. Neo Entomol 35:382–389. https://doi.org/10.1590/S1519-566X2006000300014
Monzó C, Mollá Ó, Castañera P, Urbaneja A (2008) Activity-density of Pardosa cribata in Spanish citrus orchards and its predatory capacity on Ceratitis capitata and Myzus persicae. Biol Control 54:393–402. https://doi.org/10.1007/s10526-008-9199-0
Monzó C, Sabater-Muñoz B, Urbaneja A, Castañera P (2011) The ground beetle Pseudophonus rufipes revealed as predator of Ceratitis capitata in citrus orchards. Biol Control 56:17–21. https://doi.org/10.1016/j.biocontrol.2010.09.004
Nielsen C, Vestergaard S, Harding S, Wolsted C, Eilenberg J (2006) Biological control of Strophosoma spp. (Coleoptera: Curculionidae) in greenery (Abies procera) plantations using Hyphomycetes, Biocontrol Sci Technol 16(6):583-598. https://doi.org/10.1080/09583150500532824
Ortiz-Urquiza A, Keyhani NO (2013) Action on the surface: entomopathogenic fungi versus the insect cuticle. InsEcts 4(3):357–374. https://doi.org/10.3390/insects4030357
Pereira H, Detrain C (2020) Prophylactic avoidance of hazardous prey by the ant host Myrmica rubra. InsEcts 11(7):444. https://doi.org/10.3390/insects11070444
Pla I, García de Oteyza J, Tur C, Martínez MA, Laurín MC, Alonso E, Martínez M, Martín A, Sanchis R, Navarro MC, Navarro MT, Argilés R, Briasco M, Dembilio O, Dalmau V (2021) Sterile insect technique programme against Mediterranean fruit fly in the Valencian community (Spain). InsEcts 12:415. https://doi.org/10.3390/insects12050415
Quesada-Moraga E, Ruiz-García A, Santiago-Alvarez C (2006) Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol 99(6):1955–1966. https://doi.org/10.1093/jee/99.6.1955
R Core Team (2023) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. www.r-project.org/
Reber A, Purcell J, Buechel SD, Buri P, Chapuisat M (2011) The expression and impact of antifungal grooming in ants. J Evol Biol 24:954–964. https://doi.org/10.1111/j.1420-9101.2011.02230.x
Ríos-Moreno A, Quesada-Moraga E, Garrido-Jurado I (2018) Treatments with Metarhizium brunneum BIPESCO5 and EAMa 01/58-Su strains (Ascomycota: Hypocreales) are low risk for the generalist predator Chrysoperla carnea. J Pest Sci 91:385–394. https://doi.org/10.1007/s10340-017-0905-5
Riviere C, Bethinger A, Bergez JE (2022) The effects of cover crops on multiple environmental sustainability indicators: a review. Agronomy 12(9):1–17. https://doi.org/10.3390/agron-omy12092011
Rodrigues A, Cable RN, Mueller UG, Bacci M, Pagnocca FC (2009) Antagonistic interactions between garden yeasts and microfungal garden pathogens of leafcutting ants. Antonie Leeuwenhoek 96:331–342. https://doi.org/10.1007/s10482-009-9350-7
Scheepmaker JWA, Butt TM (2010) Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biol Sci Technol 20(5):503–552. https://doi.org/10.1080/09583150903545035
Sosa-Gómez DR (2017) Chapter 13 - microbial control of soybean pest insects and mites. In: Lacey LA (ed) Microbial control of insect and mite pests: from theory to practice. Academic Press, Cambridge, Massachusetts, pp 199–208. https://doi.org/10.1016/B978-0-12-803527-6.00013-5
Vanninen I, Hokkanen H, Tyni-Juslin J (1999) Screening of field performance of entomopathogenic fungi and nematodes against cabbage root flies (Delia radicum L. and D. floralis (Fall.); Diptera, Anthomyiidae). Acta Agric Scand 49:167–183. https://doi.org/10.1080/09064719909362513
Vestergaard S, Cherry A, Keller S, Goettel M (2003) Safety of hyphomycete fungi as microbial control agents. In: Hokkanen HMT, Hajek AE (ed) Environmental iImpacts of microbial insecticides: need and methods for risk assessment. Springer, New York, pp 35–62. https://doi.org/10.1007/978-94-017-1441-9_3
Weldon C (2020) Ceratitis capitata (Mediterranean fruit fly). CABI Compendium. https://doi.org/10.1079/cabicompendium.12367
Yousef M, Quesada-Moraga E, Garrido-Jurado I (2015) Compatibility of herbicides used in olive orchards with a Metarhizium brunneum strain used for the control of preimaginal stages of tephritids in the soil. J Pest Sci 88:605–612. https://doi.org/10.1007/s10340-014-0632-0
Yousef M, Garrido-Jurado I, Ruíz-Torres M, Quesada-Moraga E (2017) Reduction of adult olive fruit fly populations by targeting preimaginals in the soil with the entomopathogenic fungus Metarhizium brunneum. J Pest Sci 90:345–354. https://doi.org/10.1007/s10340-016-0779-y
Yousef M, Alba-Ramírez C, Garrido Jurado I, Mateu J, Raya Díaz S, Valverde-García P, Quesada-Moraga E (2018) Metarhizium brunneum (Ascomycota; Hypocreales) treatments targeting olive fly in the soil for sustainable crop production. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00001
Acknowledgements
We thank M. Piquer (UJI) and A. Benages (COCALNI, Cooperativa Citrícola de Les Alqueries) for technical support and provision of the experimental orchard, respectively, and A. Nieto from the University of Córdoba for technical support in fungal detection. Financial support has been provided by the European Union’s Horizon 2020 Program for Research and Innovation grant number 818184 (FF-IPM) and PRIMA grant number 1812 (PLANT-B), and Excellence Project PROYEXCEL00808 from the Andalusian Regional Government.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Financial support has been provided by the European Union’s Horizon 2020 Program for Research and Innovation Grant Number 818184 (FF-IPM) and PRIMA Grant Number 1812 (PLANT-B), the Spanish Ministry of Science and Innovation through the Project PID2019-103844RB-I00, and the Andalusian Regional Government through the Excellence Project PROYEXCEL00808.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
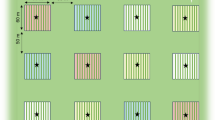
Fig. 1 Supplementary
. Aerial view of the experimental orchard with the 24 caged trees (4 × 4 × 4 m3). Half of these cages had a seeded cover of Lolium arundinaceum (green background) whereas the other half had bare soil (gray background). Three different treatments were randomly assigned to each of these covers. C was a control treatment, while A1 and A2 represented the application of either one or two treatments of M. brunneum EAMa 01/58-Su strain, respectively. (TIFF 6595 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cruz-Miralles, J., Garrido-Jurado, I., Yousef-Yousef, M. et al. Compatibility of soil application of Metarhizium brunneum and cover crops against Ceratitis capitata soil-dwelling stages. J Pest Sci (2024). https://doi.org/10.1007/s10340-023-01705-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01705-6