Abstract
As the increasing globalisation of trade generates an escalating spread of arthropod pests, eradication has gained traction as a viable approach to avoiding the growing long-term management costs. The Sterile Insect Technique (SIT) involves releasing sexually sterile insects into the wild population and has been employed for environmentally friendly eradication. Alternatively, classical biological control (CBC) comprises the importation and release of natural enemies. Although generally used for long-term management, evidence suggests a synergistic impact could be exerted on pest populations when combined with SIT, potentially improving eradication outcomes. It is possible that sterile parasitoids, which would not bear the risk of irreversible non-target impacts associated with conventional CBC releases, could be accepted by regulatory agencies as a safe option to be used as a synergistic component of eradication. We investigated the post-irradiation behaviour and fitness of the egg parasitoid Trissolcus basalis to determine whether irradiation-induced sterility may reduce its efficacy. In comparing sterile and non-sterile parasitoids, there were no observable differences in searching behaviour, no significant differences in the number of egg masses found and parasitised, nor longevity. It is possible that sterile parasitoid release could contribute to an eradication programme without detrimental effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world is facing an escalating spread of unwanted arthropod pests due to the increasing globalisation of trade, which is a key invasion pathway (Tobin et al. 2014; Liebhold et al. 2016; Suckling et al. 2019b). This has translated to a growing focus on biosecurity preparedness. For example, border surveillance utilises tools such as baited traps to monitor the potential arrival of pests at points-of-entry to enable early detection and rapid response before they become established (Poland and Rassati 2019; Fan et al. 2019). Eradication is often attempted as a response to the detection of pests that are expected to exert substantial economic and/or environmental impacts, to avoid the accumulating costs of long-term pest management (Brockerhoff et al. 2010; Suckling et al. 2012). However, cost-effective and successful eradication is generally dependent on the early detection of, and response to, pest incursions, whereby the target area of infestation remains small and localised (Pluess et al. 2012; Tobin et al. 2014). Despite increasing restrictions on the application of broad-spectrum insecticides (Handford et al. 2015), which can impact non-target organisms and effect human health, synthetic chemical pesticides continue to be widely used as the basis of eradication efforts (Hajek and Tobin 2010; Herms and McCullough 2011). However, shifting attitudes have made environmentally friendly and sustainable, and therefore socially acceptable, methods to achieve management or eradication outcomes more desirable (Suckling et al. 2017; Mankad et al. 2017; Paterson et al. 2019).
One such method is the Sterile Insect Technique (SIT), which entails the mass-rearing and subsequent repetitive release of sexually sterile conspecifics of the target pest. The most common approach is to engender sterility in males through gamma or X-ray radiation, thereby inhibiting offspring production upon mating with wild females (Klassen and Curtis 2021). The success of the SIT requires the release of sufficient numbers of sterile insects to achieve an overflooding ratio (sterile: wild insect) that results in a reduced population size in the subsequent generation (Lance and McInnis 2021). Key factors that influence the required overflooding ratio for a given target pest, and thereby the cost and feasibility of SIT application, are the intrinsic population growth rate, degree of induced sterility, and the ability of sterile males to compete for mates (Knipling 1979; Lance and McInnis 2021). Although generally applied as a constituent of area-wide integrated pest management, where pest populations are controlled within a delimited area (Hendrichs et al. 2007), SIT has also been used in eradication programmes, particularly as an early response at low target pest densities (Brockerhoff et al. 2010; Klassen and Curtis 2021). Notable examples include the eradications of Cochliomyia hominivorax (Coquerel) (Diptera: Calliphoridae) from North and Central America (Vargas-Teran et al. 2021), and Teia anartoides (Walker) (Lepidoptera: Erebidae) from Auckland, New Zealand (Suckling et al. 2007). Despite a remarkable history of successful SIT-based eradication programmes, the approach is not infallible. For instance, the eradication of C. hominivorax from Jamaica was unsuccessful due to the programme being initiated with limited data available on the population ecology of the pest in the country (Vreysen et al. 2007). Benefits of the SIT include the lack of non-target or other environmental risks, and high efficacy at low target pest densities (Nagel and Peveling 2021).
Classical biological control (CBC) is another generally environmentally benign pest control tool, which involves importing and releasing natural enemies to control target pest populations and, consequently, their impact (DeBach and Rosen 1991). Intentionally released CBC agents rarely cause population-level non-target impacts due to the prioritisation of target-specific species. Their populations are self-sustaining, and target pests do not develop resistance (van Lenteren et al. 2006; Bale et al. 2008).
The integration of biological control with SIT has received steady scientific interest since Knipling (1979) and Barclay (1987) first theorised that the combination should interact synergistically, with each component yielding a greater impact against target populations than if it were applied individually. This suggests that the integration of biological control as a component of an SIT-based eradication programme could improve efficacy and the chances of success (Horrocks et al. 2020). However, some governments institute strict regulations surrounding the release of CBC agents because of perceived irreversible risks to non-target species (Barratt et al. 2006a, 2006b; Sheppard et al. 2003), despite the rarity of population-level impacts (van Lenteren et al. 2006). Therefore, methodologies for best practice biosafety have been developed and implemented in many countries during pre-release risk assessment to assess the potential risks posed by a CBC candidate (van Lenteren et al. 2003, 2006). Consequently, the timeframe between application to permit the release of CBC agents and approval limits the prospect of safely releasing an agent as part of an early detection SIT-based eradication response at a time when eradication would remain feasible (Sheppard et al. 2003; Suckling et al. 2012).
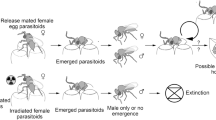
These regulatory constraints could be averted by also administering the SIT to a parasitoid CBC agent, which has been termed the “Kamikaze Wasp Technique” (KWT) (Horrocks et al. 2020). A recent study demonstrated that irradiating females of an egg parasitoid, Trissolcus basalis (Wollaston) (Hymenoptera: Scelionidae), inhibited their ability to produce offspring without evident reductions in oviposition or host mortality. If released, kamikaze wasps would not form a self-sustaining population, thereby eliminating the irreversibility of non-target risk and potentially providing a route through which biological control could be more feasibly implemented as a synergistic component of SIT-based eradication (Horrocks et al. 2021). However, SIT programmes require an assessment of post-irradiation fitness and behaviour to ensure that sterile males can compete for mates amongst the wild male population (Lance and McInnis 2021). This would similarly be crucial for investigating the feasibility of sterile parasitoid release, as they would need to retain the behaviours required to locate and attack hosts in the field.
In this study, the post-irradiation host-searching behaviour of sterile T. basalis females was investigated by quantifying their retention time in a host-contaminated arena, and by assessing their ability to locate and parasitise Nezara viridula (Linnaeus) (Hemiptera: Pentatomidae) egg masses inside a large cage. Longevity was also examined as an indicator of post-irradiation fitness (Wee et al. 2005; Whitten and Mahon 2021). This represents a model system supporting the continuing development of SIT as a potential eradication tool for the rapidly spreading pest Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) (Suckling et al. 2019a, b; Welsh et al. 2017) because its parasitoid, Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae), is congeneric to T. basalis.
Methods
Source of insects.
Insects used for experiments were sourced from established colonies of N. viridula and T. basalis at The New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand. Both colonies were maintained in a controlled temperature room (25 ± 1 °C, 16:8 h L:D). The N. viridula colony originates from adults collected on Cleome sp. at Kelmarna Gardens, Auckland (36°51′03.3″ S, 174°43′58.7″ E), on tomato plants at Ruawai (36°06′06″ S, 174°00′05″ E), and on bean plants at Mangakino (38°22′22″ S, 175°46′33″ E). The T. basalis colony was formed from the collection of parasitised N. viridula egg masses on Cleome sp. at Kelmarna Gardens.
Insect rearing
Nezara viridula were reared in plastic rearing cages (69 cm diameter × 20 cm height) and provided green beans and raw peanuts as a food source and moist cotton wool as a water source. Waxed paper, folded into a fan shape, was placed into cages containing adults as an oviposition surface. The T. basalis colony was maintained inside a nylon mesh cage (69 × 48 × 48 cm), where N. viridula egg masses were exposed to female parasitoids inside plastic vials. A drop of honey was provided as a food source. The rearing procedure for both colonies is detailed in Horrocks et al. (2021).
Host location, recognition, acceptance and longevity experiments
Experiment 1. The host location and recognition ability of sterile T. basalis females was assessed using an arena with filter paper containing footprints of its host N. viridula in the centre. This was adapted from the methodologies of Colazza et al. (1999), Peri et al. (2006, 2013) and Salerno et al. (2006). To ensure that female N. viridula were gravid, male and female pairs were collected one to two days after adult eclosion and kept in individual vented Petri dishes (90 mm diameter, 20 mm height) with a green bean, raw peanut and moist cotton wool. The mating status of each pair was checked daily, whereby pairs observed mating were labelled. Only pairs that were confirmed to have mated were used in the experiment. The day after mating finished, two gravid female N. viridula adults were placed in the centre of a circular piece of filter paper (240 mm diameter) and quickly covered by a Petri dish (55 mm diameter, 8 mm height) to confine them within this area for three hours. This ensured that the tarsi were in constant contact with the filter paper, which would absorb odours. The remaining area was untreated. A plastic box was then placed over the arena to ensure that the untreated area surrounding the centre did not become contaminated by external odour sources. A line was traced around the Petri dish with a pencil to define the treated area upon removal of the Petri dish and N. viridula.
Five T. basalis females were irradiated in glass vials (50 mm height, 11 mm diameter) at 150 Gy and then transferred to plastic vials (57 mm height, 22 mm diameter) for 24 h with a drop of honey (~ 50 μL) (see Horrocks et al. (2021) for details of irradiation technique). A single two- to six-day old naïve irradiated T. basalis female was gently released into the centre of the treated area, and any arrestment response to the host-contaminated surface, as characterised by a sudden decrease in linear speed and increase in turning rate (Colazza et al. 1999), was recorded. The total time the parasitoid spent either arrested or antennating inside the host-contaminated area of the filter paper was recorded (retention time) to assess host location and recognition ability inside a host patch. Parasitoids that flew away before walking at least 5 mm were excluded from analysis as it was necessary to allow time for antennal contact on the host-contaminated surface. All five irradiated T. basalis females were released individually onto the arena, and the process was replicated seven times (N = 35 parasitoids), with a new arena being prepared for each replicate. This was repeated using non-irradiated females as a positive control to assess whether irradiation impacted host-location behaviour and host patch residence ability. For the negative controls, the method was also repeated using an identical arena, but without N. viridula being constrained within the 55 mm diameter treatment area, to confirm no contamination of the arena from other sources. The control groups consisted of the same number of replicates as the treatment group.
Experiment 2. A lab-based oviposition cage assay was conducted, adapted from Amalin et al. (2005), Chailleux et al. (2012) and Sadek et al. (2010), to investigate the potential difference in host-location and host-acceptance ability between irradiated and non-irradiated T. basalis females in a larger space. Twenty leaves (2 to 3 cm long) were removed from potted tomato plants, which are a favoured host of N. viridula for both food and oviposition (Smaniotto and Panizzi 2015), and separated with the adaxial surface facing down into four Petri dishes (90 mm diameter, 20 mm height) containing moist cotton wool to prevent desiccation. Two female N. viridula were then placed into each Petri dish for three hours to allow the deposition of footprint odours onto the tomato leaves. Forceps were used to gently encourage the N. viridula to walk around the area of the Petri dish every hour, ensuring host contamination of each leaf. A nylon mesh oviposition cage (120 cm high × 90 cm long × 90 cm wide) was set up inside a controlled temperature room (25 ± 1 °C, 16:8 h L:D). Immediately after N. viridula exposure, the adaxial surfaces of the 20 tomato leaves were stuck to the ceiling of the mesh cage in a grid, ensuring even spacing, using double-sided tape. This allowed the host-contaminated abaxial surfaces of the leaves to be fully exposed. Four N. viridula egg masses, no older than 24 h and stored at 10 °C for up to three days (Haye et al. 2020), were individually stuck to pieces of card, which were then cut as close to the edge of the masses as possible. One egg mass was stuck to the underside of each of four tomato leaves, randomly selected out of the 20 leaves on the cage ceiling, using a piece of double-sided tape smaller than the surface area of the egg mass. A vial containing four 150 Gy irradiated female T. basalis, no older than four days and rested for 24 h, was placed at the centre of the floor of the cage and opened to release the wasps. For the first two hours after their release, parasitoids were observed for 10 min periods every 30 min to ascertain the time taken to locate and parasitise N. viridula egg masses. The number of wasps that located the same egg mass was also recorded during the observation period, as it was possible that all wasps may locate egg masses without all egg masses being located. Observations ceased if parasitoids located and parasitised all egg masses before the two hours were completed.
The N. viridula egg masses were collected 24 h after parasitoid release and individually placed into vials (57 mm height, 22 mm diameter) in a controlled temperature room (25 ± 1 °C, 16:8 h L:D). Those egg masses that were not found by parasitoids during the observation period were subsequently observed daily for signs of parasitism to confirm whether parasitoids successfully located the host. Because irradiated T. basalis females are sterile and usually kill the host egg without developing progeny (Cusumano et al. 2018; Horrocks et al. 2021), parasitism of an egg mass was confirmed if it contained eggs without developing N. viridula nymphs. Therefore, host nymphal development was also recorded for eggs that turned pink (Bundy and McPherson 2000). Infertile eggs maintain a pale colour without change (Borges et al. 1999) and were consequently excluded from analysis because it was not possible to ascertain what caused the absence of host nymphal development. This experiment was replicated ten times (N = 40 egg masses and 40 parasitoids). It was repeated with the same number of replicates for non-irradiated female T. basalis to ascertain whether there was a post-irradiation difference in host-location ability within the cages. For the non-irradiated positive control, parasitism was confirmed through progeny development (eggs turn black) and lack of host nymphal development. Due to an unexpected low degree of T. basalis offspring emergence from 150 Gy irradiated parasitoids, which completely inhibited offspring emergence in a previous dose–response study on this species (Horrocks et al. 2021), this was repeated with 180 Gy irradiated females. This also provided dosimetry analysis extending to a higher irradiation dose than had previously been investigated for T. basalis. The mean number of eggs in masses exposed in cages were 60.95, 53.10 and 58.90 for the 150 Gy, 180 Gy and non-irradiated treatments, respectively.
As a negative control, this experiment was repeated without releasing parasitoids into the oviposition cage, and replicated ten times. This demonstrated the degree of N. viridula egg fertility and nymphal development in the absence of parasitoids to confirm parasitism as the cause of host death.
Experiment 3. Experiments were run without host eggs to ascertain the longevity of irradiated parasitoids. Five three-day-old female T. basalis were irradiated at 180 Gy inside a glass vial (50 mm height, 11 mm diameter). Irradiated parasitoids were then returned to the laboratory (25 ± 1 °C, 16:8 h L:D) and transferred into a plastic vial (57 mm height, 22 mm diameter) with a drop of honey (~ 50 μL) for food, which was replenished every three to four days. This was replicated eight times (N = 40 parasitoids). The vials were observed daily until the last parasitoid died, and the number of days survived from initial emergence was recorded each time an individual died. Parasitoids that drowned in the honey were excluded from the analysis. This process was repeated eight times with non-irradiated females as a control.
Statistical analysis. Poisson generalised linear models (GLM) were used to compare irradiated and non-irradiated T. basalis females for time spent either arrested or antennating on the inner contaminated area of arenas. Poisson GLMs were also applied when comparing time spent on control and host-contaminated arenas by both irradiated and non-irradiated parasitoids, and for comparing irradiated and non-irradiated parasitoids on control arenas. Cox proportional hazards models were used to assess the potential differences in the accumulation of N. viridula egg masses found and attacked by 0 Gy, 150 Gy and 180 Gy parasitoids after two hours in oviposition cages, and when comparing each irradiated parasitoid group with the control group. These models were also applied for the same comparison between irradiated (150 Gy + 180 Gy) and control parasitoids, and between the two irradiated groups. Binomial GLMs were used to examine the relationship between irradiation dose and the mean percentage of N. viridula eggs parasitised and the percentage of egg masses parasitised after parasitoids spent 24 h inside oviposition cages. These were also used when assessing the other group comparisons for the same variables. Infertile eggs were excluded from these analyses because they were not used to confirm parasitism. Finally, a Poisson GLM was applied to compare longevity between irradiated and sterile T. basalis females. All GLMs were subject to analysis of variance (ANOVA), and presented statistics were produced in R (version 3.6.0; R Foundation for Statistical Computing, Australia). R packages used comprised ggplot2 (Wickham 2016) for plots, and rms (Harrell 2021) and lme4 (Bates et al. 2015) for analyses.
Results
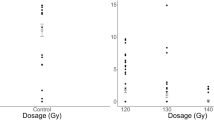
Experiment 1. The time spent arrested and antennating within the central host-contaminated portion of the filter paper arenas did not differ between 150 Gy irradiated and non-irradiated T. basalis females (Fig. 1). Mean arrestment and antennation time within the host-contaminated area was 52.63 s for irradiated and 51.18 s for non-irradiated T. basalis. There is no statistical evidence to suggest a difference (F1, 57 = 0.03, P = 0.868). Mean arrestment and antennation time for the irradiated control group, where the central portion of the arena was not host-contaminated, was 3.99 s, which was significantly shorter than for the irradiated treatment group (F1, 56 = 121.10, P < 0.001). A similar pattern was observed when comparing the non-irradiated treatment and control groups (F1, 59 = 68.14, P < 0.001). There was also no discernible difference between the irradiated and non-irradiated control groups (F1, 58 = 1.25, P = 0.268).
Time spent arrested and antennating within the inner host-contaminated area of filter paper arenas by 150 Gy irradiated and non-irradiated female Trissolcus basalis. Controls represent arenas that were not exposed to female Nezara viridula. Boxes depict the interquartile range, with whiskers being 1.5 times the interquartile range. Squares represent means
Experiment 2. During the two hour observation periods following parasitoid release into oviposition cages, parasitoids irradiated at 0, 150 and 180 Gy found and attacked 23 host egg masses (from a total of 40) (Fig. 2). Although the cumulative number of host egg masses parasitised differed slightly when considering each observation period, this was not statistically significant when comparing all three groups (concord = 0.74, SE = 0.07, df = 2.00, P = 0.200), or either irradiation group to the control (150 Gy: coef = -0.56, SE = 0.46, Z = -1.21, CI = [0.23, 1.41], P = 0.226; 180 Gy: coef = -0.95, SE = 0.51, Z = -1.86, CI = [0.14, 1.05], P = 0.062). There was also no significant difference in the cumulative number of host masses attacked when comparing non-irradiated and irradiated (150 + 180 Gy) parasitoids (coef = 0.74, SE = 0.42, Z = 1.77, CI = [0.92, 4.72], P = 0.077), or when comparing 150 and 180 Gy irradiated parasitoids (coef = − 0.40, SE = 0.49, Z = − 0.80, CI = [0.26, 1.77], P = 0.421).
The cumulative number of Nezara viridula egg masses found by irradiated and non-irradiated Trissolcus basalis females over a two hour period following their release into oviposition cages. Observation periods represent 10 min observation windows every 30 min. Circles, triangles and squares represent 0, 150 and 180 Gy irradiated parasitoids, respectively
There was no significant difference in the relationship between T. basalis radiation dose and mean percentage of N. viridula egg masses located and parasitised in large cages after 24 h (F2, 27 = 2.45, P = 0.106) (Fig. 3). The means for 0, 150, and 180 Gy were 95, 100, and 90% of egg masses located and parasitised, respectively, with no dose–response relationship observed. Additionally, there was no difference in the mean percentage of egg masses parasitised between non-irradiated and irradiated (150 + 180 Gy) parasitoids (F1, 28 = 0.00, P = 1.000), with the latter also being 95%. Considering all N. viridula eggs inside the cages, there was no significant difference in percent parasitism after 24 h between the irradiation doses tested (F2, 117 = 1.40, P = 0.250); thus, no dose–response relationship was observed (Fig. 4). There was also no significant difference in percent parasitism between non-irradiated and irradiated parasitoids (F1, 118 = 0.54, P = 0.463). For the negative control, where no parasitoids were released into the cages, the percentage of eggs that contained developing N. viridula nymphs was 97.23%. The mean percentage of N. viridula nymph development inside eggs was 97.08% amongst egg masses.
Experiment 3. The longevity of naïve T. basalis females did not differ significantly between the irradiated and non-irradiated groups (F1, 60 = 0.00, P = 0.967) (Fig. 5). Mean longevity was very similar between the two groups, being 50.55 days for non-irradiated parasitoids and 50.68 days for irradiated parasitoids. However, the non-irradiated T. basalis expressed a wider range of longevity values.
Discussion
Key findings and framework
Sterile T. basalis females, irradiated at 180 Gy, did not show significant differences in host location, recognition, acceptance or longevity compared with non-irradiated females in lab-scale experiments. This indicates that sterile parasitoids could contribute to an SIT-based eradication programme without the need to compensate for any effects on host-finding behaviour and fitness. However, due to the transient nature of sterile parasitoid release, conventional self-sustaining CBC would be required for long term control if eradication of the target pest were to become infeasible. Scrutinising sterile female parasitoid host-finding behaviour and fitness is analogous to competitive fitness assessments in conventional SIT efficacy research for commonly targeted Dipteran and Lepidopteran pests, whereby sterile insects must retain the ability to compete for wild mates (Parker et al. 2021). A reduction in fitness can translate to the need for a higher overflooding ratio, which can influence the cost-effectiveness of a proposed SIT programme (Whitten and Mahon 2021).
Nevertheless, because the KWT is designed to facilitate the contribution of biological control agents to early-response eradication efforts, releases would be focused upon low density pest infestations within a highly localised area (Pluess et al. 2012; Liebhold et al. 2016). Releasing an adequate number of sterile parasitoids for sufficient impact against the target pest is therefore expected to be achievable and cost-effective (Brockerhoff et al. 2010). The local-scale nature of these releases should also alleviate concern surrounding ephemeral non-target impacts that can potentially occur from inundative parasitoid releases (van Lenteren et al. 2006). However, population modelling and field trials assessing the mass-release of sterile parasitoids are required to refine our understanding of required overflooding ratios and how potential behavioural and fitness impacts of irradiation may impact their efficacy.
Previous studies involving the irradiation of parasitoids, though not for application in pest management, have made varying secondary observations related to post-irradiation fitness. Tillinger et al. (2004) found that 48 and 96 Gy irradiated female Glyptapanteles liparidis (Bouché) (Hymenoptera: Braconidae) expressed a significant reduction in oviposition compared to non-irradiated females but no change in longevity. Conversely, Soller and Lanzrein (1996) observed no difference in oviposition from 146 Gy irradiated Chelonus inanitus (Linnaeus) (Hymenoptera: Braconidae). However, no studies have systematically investigated the effects of irradiation-induced sterility on parasitoid fitness, host-searching behaviour, and host location, which are likely to be critical to success with the KWT. The latter two are of particular importance when considering the efficacy of sterile parasitoid release because irradiated biological control agents must retain the ability to detect host-kairomones, and the associated searching behaviours, to locate and parasitise their hosts (Colazza et al. 1999; Peri et al. 2013).
Post-irradiation host-finding
Because host eggs provide only a small window of opportunity for parasitism (Vinson 1998, 2009) and do not leave behind a chemical trail like with mobile hosts, egg parasitoids generally rely upon indirect host cues, particularly adult female chemical footprints and oviposition-induced plant synomones, to locate target egg masses (Colazza et al. 2004, 2009; Fatouros et al. 2008; Conti et al. 2010). These cues stimulate characterised arrestment and searching behaviours in egg parasitoids, which is a valuable system for investigating host-finding ability. The arena retention time experiment examines irradiated parasitoids’ arrestment and searching behaviours in response to contact with host chemical footprints on filter paper (Colazza et al. 1999; Peri et al. 2013). The substantial difference in arena retention time between female T. basalis exposed to arenas with a host-contaminated area and those without, suggests that they responded to host-related chemical cues with searching behaviour and arrestment. The similar mean arrestment and antennation time observed between 150 Gy irradiated and non-irradiated parasitoids exposed to host-contaminated arenas indicates that irradiation did not affect their ability to detect chemicals associated with the host footprints and subsequently initiate arrestment and searching behaviours. This is a crucial observation for the feasibility of developing the KWT because kairomone mediated host-searching behaviour is fundamental to the ability of parasitoids to locate and parasitise their hosts in the field (Fatouros et al. 2008; Peri et al. 2013).
The number of egg masses located and parasitised within oviposition cages provides insight into whether the arena results translate to the ability of irradiated parasitoids to locate host egg masses on host plant material and at a larger scale (Sadek et al. 2010; Chailleux et al. 2012). We placed egg masses on tomato leaves exposed to female N. viridula inside the cage because Trissolcus parasitoids respond to synomones released by plants on which their pentatomid hosts are active (Colazza et al. 2004; Frati et al. 2013). This consequently represented a more realistic host-searching environment. The lack of statistically significant differences in the cumulative number of egg masses found and parasitised within 2 h of parasitoid release between irradiated and non-irradiated parasitoids suggests that irradiation did not impede host-location ability inside the cages. No difference in the total number of egg masses parasitised between irradiated and non-irradiated parasitoids after 24 h inside cages also supports this interpretation.
This is the first study to evaluate the potential effect of irradiation on chemically mediated host-searching in parasitoids. However, previous studies on post-irradiation competitive fitness for commonly targeted pest species have analogously investigated the impact of irradiation on responsiveness to sex pheromones, with varying results. White and Hutt (1975) found that field traps baited with synthetic female Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) sex pheromone expressed lower catch-rates of irradiated conspecific males compared to untreated males, suggesting that irradiation depressed the olfaction-mediated mate-finding system. However, a similar study on Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) observed no impact of irradiation dose on male responsiveness to synthetic female sex pheromone (Stimmann et al. 1972). More recent inquiries have also found varying results amongst different Diptera and Lepidoptera species regarding irradiated insect responsiveness to pheromones (Waldvogel et al. 1982; Moreno et al. 1991; Stringer et al. 2013). For T. basalis, our results support further development of the KWT as a potential eradication component by demonstrating that host-searching ability in lab-scale experiments is maintained in irradiated females, which is crucial for efficacy. However, the varying results from previous studies on the impact of irradiation on pheromone responsiveness suggest that our observations may not necessarily be the same in other parasitoid species, and chemically mediated host-searching behaviour would need to be examined on a case-by-case dose–response basis for any candidate pest-parasitoid system. These time-consuming investigations would require proactive consideration to contribute to SIT-based eradication programmes, which demand a rapid response at low target pest densities during the early stages of invasion (Brockerhoff et al. 2010; Tobin et al. 2014).
Post-irradiation fitness
Parasitism rate is not always considered a sufficient indicator of parasitoid fitness because of its disregard for the impact of variables such as host distribution and parasitoid longevity (van Baalen and Hemerik 2008). However, parasitism rate is commonly used as an indicator for efficacy and relates to lifetime reproductive success since it is a prerequisite for offspring emergence (Urrutia et al. 2007; Colinet et al. 2007; Zhou et al. 2014; Rossi Stacconi et al. 2015). Fully sterile female parasitoids do not produce emerging offspring (Horrocks et al. 2021). Because the same number of host egg masses were exposed to parasitoids in each replicate, our parasitism results are discussed within the context of post-irradiation fitness. No significant differences were observed amongst the non-irradiated, 150, and 180 Gy parasitoid groups for the percentage of both egg masses and total eggs parasitised. Irradiation-induced sterility, therefore, had no impact on the percentage of hosts attacked and killed within a large searching area.
Previous studies involving the irradiation of parasitoids did not assess parasitism within large searching areas, with hosts being directly exposed to parasitoids. However, Tunçbilek et al. (2009) found that the mean number of host eggs parasitised by Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae) substantially decreased with an increase in irradiation dose. Tillinger et al. (2004) also observed that 48 and 96 Gy irradiated G. liparidis females oviposited significantly fewer eggs than control wasps. However, it is not clear to what extent this reduction may impact the parasitism rate because this parasitoid deposits multiple eggs into each host larvae (Schopf and Steinberger 1996). Conversely, irradiation-induced sterility in C. inanitus females does not affect oviposition behaviour (Soller and Lanzrein 1996). Our results and previous studies demonstrate that parasitism capacity in sterile parasitoids may vary amongst species. This highlights the importance of investigating parasitoid efficacy for the potential field application of sterile parasitoids, particularly as a potential eradication component. Required sterile release overflooding ratios would be influenced by parasitoid efficacy and inform the fiscal and ecological feasibility of the rapid response necessary for improving the chances of a successful SIT-based eradication programme (Lance and McInnis 2021; Whitten and Mahon 2021).
Longevity is a commonly considered measure of fitness for sterile insects because a reduction would result in lower reproductive fitness than wild conspecifics. Therefore, the need for higher sterile release overflooding ratios leads to more significant expense (Wee et al. 2005; Whitten and Mahon 2021). This would bear similar importance for efficacy assessments for sterile parasitoids, as a reduction in longevity could reduce the time available for oviposition. Our results show that the longevity of irradiated and non-irradiated parasitoids with a continuous food source was almost identical. However, Horrocks et al. (2021) found that when offered N. viridula egg masses throughout their lifetimes, irradiated T. basalis females lived significantly longer and attacked a similar average number of host eggs over a more extended period. This likely represented a fitness trade-off between fecundity and longevity, well-known amongst insects. This study suggests that irradiation may not impact field performance for this species. A significant decrease in longevity is generally reported with irradiation-induced sterility in insects (Whitten and Mahon 2021). The only other study involving irradiation-induced sterility in parasitoids that assessed longevity demonstrated that irradiating G. liparidis did not significantly reduce longevity (Tillinger et al. 2004). However, considering the limited knowledge of the effects of irradiation on longevity in parasitoids and the varying results amongst conventionally-targeted insect orders (El-Kholy 2009; Draz et al. 2016; Ali et al. 2016), it would be essential to implement it as a component of quality assessments to inform potential feasibility of their synergistic contribution to SIT-based eradication.
Conclusion
This is the first experimental study systematically investigating sterile parasitoids’ post-irradiation behaviour and fitness. Previous work demonstrated that irradiation could eliminate the production of offspring in T. basalis females, suggesting a negligible risk of ongoing non-target impacts if they were to be released as classical biocontrol agents. Therefore, the proposal to release sterile parasitoids could potentially circumvent the normally stringent regulations surrounding their importation and release since there is no possibility of a self-sustaining population.
Conventional introductions of new biocontrol agents face concerns over irreversible non-target risks. Still, our one-use approach could potentially improve the feasibility of biological control as a synergistic component of a rapid response, and especially an SIT-based eradication. Building on this, the current study demonstrated no significant impacts of irradiation on various performance measures we assessed in T. basalis females, which provides further support for the feasibility of the KWT. However, sterile parasitoid efficacy must next be investigated at the field-cage and open-field scales to provide convincing evidence of field performance following the frequently applied stepwise framework for conventional post-irradiation efficacy assessments (Simmons et al. 2010) and to substantiate the potential for a new arthropod eradication tool.
Authors’ contributions
KJH, DMS, GAA, and GIH conceived and designed the study. KJH and GAA conducted experiments and collected data. KJH and GIH analysed the data. GAA contributed materials and tools. KJH wrote the manuscript. All authors read and approved the manuscript.
Data Availability
All data will be made available upon request.
References
Ali A, Rashid MA, Huang QY, Lei C-L (2016) Effect of UV-A radiation as an environmental stress on the development, longevity, and reproduction of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Environ Sci Pollut Res 23:17002–17007. https://doi.org/10.1007/s11356-016-6865-0
Amalin DM, Peña JE, Duncan RE (2005) Effects of host age, female parasitoid age, and host plant on parasitism of Ceratogramma etiennei (Hymenoptera: Trichogrammatidae). Fla Entomol 88:77–82. https://doi.org/10.1653/0015-4040(2005)088[0077:EOHAFP]2.0.CO;2
Bale JS, van Lenteren JC, Bigler F (2008) Biological control and sustainable food production. Phil Trans R Soc Lond B 363:761–776. https://doi.org/10.1098/rstb.2007.2182
Barclay HJ (1987) Models for pest control: complementary effects of periodic releases of sterile pests and parasitoids. Theor Popul Biol 32:76–89. https://doi.org/10.1016/0040-5809(87)90041-4
Barratt BIP, Blossey B, Hokkanen HM (2006a) Post-release evaluation of non-target effects of biological control agents. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. CABI Publishing, Wallingford, pp 166–186
Barratt BIP, Moeed A, Malone LA (2006b) Biosafety assessment protocols for new organisms in New Zealand: can they apply internationally to emerging technologies? Environ Impact Assess Rev 26:339–358. https://doi.org/10.1016/j.eiar.2005.11.008
Bates D, Machler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Borges M, Costa MLM, Sujii ER et al (1999) Semiochemical and physical stimuli involved in host recognition by Telenomus podisi (Hymenoptera: Scelionidae) toward Euschistus heros (Heteroptera: Pentatomidae). Physiol Entomol 24:227–233. https://doi.org/10.1046/j.1365-3032.1999.00136.x
Brockerhoff EG, Liebhold AM, Richardson B, Suckling DM (2010) Eradication of invasive forest insects: concepts, methods, costs and benefits. NZ J Forestry Sci 40:118–135
Bundy CS, McPherson RM (2000) Morphological examination of stink bug (Heteroptera: Pentatomidae) eggs on cotton and soybeans, with a key to genera. Ann Entomol Soc Am 93:616–624. https://doi.org/10.1603/0013-8746(2000)093[0616:MEOSBH]2.0.CO;2
Chailleux A, Desneux N, Seguret J et al (2012) Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 7:e48068. https://doi.org/10.1371/journal.pone.0048068
Colazza S, Salerno G, Wajnberg E (1999) Volatile and contact chemicals released by Nezara viridula (Heteroptera:Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol Control 16:310–317. https://doi.org/10.1006/bcon.1999.0763
Colazza S, McElfresh S, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
Colazza S, Peri E, Salerno G, Conti E (2009) Host searching by egg parasitoids: exploitation of host chemical cues. In: Consoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, Netherlands, pp 97–147
Colinet H, Boivin G, Hance Th (2007) Manipulation of parasitoid size using the temperature-size rule: fitness consequences. Oecologia 152:425–433. https://doi.org/10.1007/s00442-007-0674-6
Conti E, Salerno G, Leombruni B et al (2010) Short-range allelochemicals from a plant–herbivore association: a singular case of oviposition-induced synomone for an egg parasitoid. J Exp Biol 213:3911–3919. https://doi.org/10.1242/jeb.045922
Cusumano A, Duvic B, Jouan V et al (2018) First extensive characterization of the venom gland from an egg parasitoid: structure, transcriptome and functional role. J Insect Physiol 107:68–80
DeBach P, Rosen D (1991) Biological control by natural enemies, 2nd edn. Cambridge University Press, Cambridge
Draz KA, Tabikha RM, El-Aw MA, Darwish HF (2016) Impact of gamma radiation doses on sperm competitiveness, fecundity and morphometric characters of peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephiritidae). J Radiat Res Appl Sci 9:352–362. https://doi.org/10.1016/j.jrras.2016.05.004
El-Kholy EMS (2009) Biological and biochemical effects of vitamin “c” on the normal and irradiated mediterranean fruit fly, Ceratitis capitata (wied.). J Radiat Res Appl Sci 2:197–212
Fan J, Denux O, Courtin C et al (2019) Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J Pest Sci 92:281–297. https://doi.org/10.1007/s10340-018-0997-6
Fatouros NE, Dicke M, Mumm R et al (2008) Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol 19:677–689. https://doi.org/10.1093/beheco/arn011
Frati F, Salerno G, Conti E (2013) Cabbage waxes affect Trissolcus brochymenae response to short-range synomones. Insect Sci 20:753–762. https://doi.org/10.1111/j.1744-7917.2012.01575.x
Hajek AE, Tobin PC (2010) Micro-managing arthropod invasions: eradication and control of invasive arthropods with microbes. Biol Invasions 12:2895–2912. https://doi.org/10.1007/s10530-010-9735-6
Handford CE, Elliott CT, Campbell K (2015) A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr Environ Assess Manag 11:525–536. https://doi.org/10.1002/ieam.1635
Harrell FE (2021) Rms: Regression Modeling Strategies. R package version 6.2–0. Springer, Switzerland
Haye T, Moraglio ST, Stahl J et al (2020) Fundamental host range of Trissolcus japonicus in Europe. J Pest Sci 93:171–182. https://doi.org/10.1007/s10340-019-01127-3
Hendrichs J, Kenmore P, Robinson AS, Vreyson MJB (2007) Area-wide integrated pest management (AW-IPM): principles, practice and prospects. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests. Springer, Netherlands, pp 3–34
Herms DA, McCullough DG (2011) Pesticides and insect eradication. In: Simberloff D, Rejmánek M (eds) Encyclopedia of invasive introduced species. University of California Press, Berkely, California, pp 528–535
Horrocks KJ, Avila GA, Holwell GI, Suckling DM (2020) Integrating sterile insect technique with the release of sterile classical biocontrol agents for eradication: is the Kamikaze Wasp Technique feasible? Biocontrol. https://doi.org/10.1007/s10526-020-09998-7
Horrocks KJ, Avila GA, Holwell GI, Suckling DM (2021) Irradiation-induced sterility in an egg parasitoid and possible implications for the use of biological control in insect eradication. Sci Rep 11:12326. https://doi.org/10.1038/s41598-021-91935-4
Klassen W, Curtis CF (2021) History of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 3–38
Knipling EF (1979) The basic principles of insect population suppression and management. US Department of Agriculture, Washington
Lance DR, McInnis DO (2021) Biological basis of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 69–94
Lenteren JCV, Babendreier D, Bigler F et al (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. Biocontrol 48:3–38
Liebhold AM, Berec L, Brockerhoff EG et al (2016) Eradication of invading insect populations: from concepts to applications. Annu Rev Entomol 61:335–352. https://doi.org/10.1146/annurev-ento-010715-023809
Mankad A, Loechel B, Measham PF (2017) Psychosocial barriers and facilitators for area-wide management of fruit fly in southeastern Australia. Agron Sustain Dev 37:67. https://doi.org/10.1007/s13593-017-0477-z
Moreno DS, Sanchez M, Robacker DC, Worley J (1991) Mating competitiveness of irradiated mexican fruit fly (Diptera: Tephritidae). J Econ Entomol 84:1227–1234. https://doi.org/10.1093/jee/84.4.1227
Nagel P, Peveling R (2021) Environment and the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 499–519
Parker AG, Vreysen MJB, Bouyer J, Calkins CO (2021) Sterile insect quality control/assurance. In: Robinson AS, Hendrichs J (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 399–440
Paterson G, Perry GLW, Walker JTS, Suckling DM (2019) Peri-urban community attitudes towards codling moth trapping and suppression using the sterile insect technique in New Zealand. InSects 10:335. https://doi.org/10.3390/insects10100335
Peri E, Sole AS, Wajnberg E, Colazza S (2006) Effect of host kairomones and oviposition experience on the arrestment behavior of an egg parasitoid. J Exp Biol 209:3629–3635. https://doi.org/10.1242/jeb.02416
Peri E, Frati F, Salerno G et al (2013) Host chemical footprints induce host sex discrimination ability in egg parasitoids. PLoS ONE 8:e79054. https://doi.org/10.1371/journal.pone.0079054
Pluess T, Jarošík V, Pyšek P et al (2012) Which factors affect the success or failure of eradication campaigns against alien species? PLoS ONE 7:e48157. https://doi.org/10.1371/journal.pone.0048157
Poland TM, Rassati D (2019) Improved biosecurity surveillance of non-native forest insects: a review of current methods. J Pest Sci 92:37–49. https://doi.org/10.1007/s10340-018-1004-y
Rossi Stacconi MV, Buffington M, Daane KM et al (2015) Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol Control 84:28–35. https://doi.org/10.1016/j.biocontrol.2015.02.003
Sadek MM, Hansson BS, Anderson P (2010) Does risk of egg parasitism affect choice of oviposition sites by a moth? A field and laboratory study. Basic Appl Ecol 11:135–143. https://doi.org/10.1016/j.baae.2009.09.003
Salerno G, Conti E, Peri E et al (2006) Kairomone involvement in the host specificity of the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Eur J Entomol 103:311–318. https://doi.org/10.14411/eje.2006.040
Schopf A, Steinberger P (1996) The influence of the endoparasitic wasp Glyptapanteles lyparidis (Hymenoptera: Braconidae) on the growth, food consumption, and food utilization of its host larva, Lymantria dispar (Lepidoptera: Lymantriidae). Eur J Entomol 93:555–568
Sheppard AW, Hill R, DeClerch-Floate RA et al (2003) A global review of risk-benefit-cost analysis for the introduction of classical biological control agents against weeds: a crisis in the making? Biocont News Info 24:91N-108N. https://doi.org/10.1016/S0960-9822(97)70976-X
Simmons GS, Suckling DM, Carpenter JE et al (2010) Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J Appl Entomol 134:261–273. https://doi.org/10.1111/j.1439-0418.2009.01438.x
Smaniotto LF, Panizzi AR (2015) Interactions of selected species of stink bugs (Hemiptera: Heteroptera: Pentatomidae) from leguminous crops with plants in the neotropics. Fla Entomol 98:7–17. https://doi.org/10.1653/024.098.0103
Soller M, Lanzrein B (1996) Polydnavirus and venom of the egg-larval parasitoid Chelonus inanitus (Braconidae) induce developmental arrest in the prepupa of its host Spodoptera littoralis (Noctuidae). J Insect Physiol 42:471–481. https://doi.org/10.1016/0022-1910(95)00132-8
Stimmann MW, Wole WW, Toba HH (1972) Pheromone response of gamma-irradiated cabbage loopers in the field. J Econ Entomol 65:1496–1498. https://doi.org/10.1093/jee/65.5.1496
Stringer LD, Sullivan NJ, Sullivan TES et al (2013) Attractiveness and competitiveness of irradiated light brown apple moths. Entomol Exp Appl 148:203–212. https://doi.org/10.1111/eea.12096
Suckling DM, Barrington AM, Chhagan A et al (2007) Eradication of the Australian painted apple moth Teia anartoides in New Zealand: trapping, inherited sterility, and male competitiveness. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests. Springer, Netherlands, pp 603–616
Suckling DM, Tobin PC, McCullough DG, Herms DA (2012) Combining tactics to exploit Allee effects for eradication of alien insect populations. J Econ Entomol 105:1–13. https://doi.org/10.1603/EC11293
Suckling DM, Conlong DE, Carpenter JE et al (2017) Global range expansion of pest Lepidoptera requires socially acceptable solutions. Biol Invasions 19:1107–1119. https://doi.org/10.1007/s10530-016-1325-9
Suckling D, Cristofaro M, Roselli G et al (2019a) The competitive mating of irradiated brown marmorated stink bugs, Halyomorpha halys, for the Sterile Insect Technique. InSects 10:411. https://doi.org/10.3390/insects10110411
Suckling DM, Stringer LD, Baird DB, Kean JM (2019b) Will growing invasive arthropod biodiversity outpace our ability for eradication? Ecol Appl. https://doi.org/10.1002/eap.1992
Tillinger NA, Hoch G, Schopf A (2004) Effects of parasitoid associated factors of the endoparasitoid Glyptapanteles liparidis (Hymenoptera: Braconidae). Eur J Entomol 101:243–249. https://doi.org/10.14411/eje.2004.033
Tobin PC, Kean JM, Suckling DM et al (2014) Determinants of successful arthropod eradication programs. Biol Invasions 16:401–414. https://doi.org/10.1007/s10530-013-0529-5
Tunçbilek AS, Canpolat U, Ayvaz A (2009) Effects of gamma radiation on suitability of stored cereal pest eggs and the reproductive capability of the egg parasitoid Trichogramma evanescens (Trichogrammatidae: Hymenoptera). Biocontrol Sci Techn 19:179–191. https://doi.org/10.1080/09583150902790269
Urrutia CMA, Wade MR, Phillips CB, Wratten SD (2007) Influence of host diet on parasitoid fitness: unravelling the complexity of a temperate pastoral agroecosystem. Entomol Exp Appl 123:63–71. https://doi.org/10.1111/j.1570-7458.2007.00526.x
van Baalen M, Hemerik L (2008) Parasitoid fitness: from a simple idea to an intricate concept. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioural ecology of insect parasitoids: from theoretical approaches to field applications. Blackwell Publishing Ltd, Massachusetts, pp 31–50
van Lenteren JC, Bale J, Bigler F et al (2006) Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol 51:609–634. https://doi.org/10.1146/annurev.ento.51.110104.151129
Vargas-Teran M, Hofmann HC, Tweddle NE (2021) Impact of screwworm eradication programmes using the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 629–650
Vinson SB (1998) The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96. https://doi.org/10.1006/bcon.1997.0601
Vinson SB (2009) Nutritional ecology of insect egg parasitoids. In: Consoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, Netherlands, pp 25–55
Vreysen MJB, Gerardo-Abaya J, Cayol JP (2007) Lessons from area-wide integrated pest management (AW-IPM) programmes with an SIT component: an FAO/IAEA perspective. Area-wide control of insect pests. Springer, Netherlands, pp 723–744
Waldvogel MG, Mastro VC, Collison CH, Cameron EA (1982) Evaluation of pheromone-mediated responsiveness of laboratory-reared irradiated, laboratory-reared nonirradiated, and feral male gypsy moths. Environ Entomol 11:351–354. https://doi.org/10.1093/ee/11.2.351
Wee SL, Suckling DM, Burnip GM et al (2005) Effects of substerilizing doses of gamma radiation on adult longevity and level of inherited sterility in Teia anartoides (Lepidoptera: Lymantriidae). J Econ Entomol 98:732–738. https://doi.org/10.1603/0022-0493-98.3.732
Welsh TJ, Stringer LD, Caldwell R et al (2017) Irradiation biology of male brown marmorated stink bugs: is there scope for the sterile insect technique? Int J Radiat Biol 93:1357–1363. https://doi.org/10.1080/09553002.2017.1388547
White LD, Hutt RB (1975) Codling moth: catches of irradiated and untreated laboratory-reared and native males in synthetic sex attractant traps. J Econ Entomol 68:449–450. https://doi.org/10.1093/jee/68.4.449
Whitten M, Mahon R (2021) Misconceptions and constraints driving oppurtunities. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile Insect Technique: principles and practice in area-wide integrated pest management, 2nd edn. Springer, Netherlands, pp 601–626
Wickham H (2016) Ggplot2: Elegant Graphics for Data Analysis. Springer, Newyork
Zhou Y, Abram PK, Boivin G, Brodeur J (2014) Increasing host age does not have the expected negative effects on the fitness parameters of an egg parasitoid. Entomol Exp Appl 151:106–111. https://doi.org/10.1111/eea.12173
Acknowledgements
We thank Anne Barrington (The New Zealand Institute for Plant and Food Research Limited (PFR)) for maintaining the N. viridula colony used for this study and Frances MacDonald (PFR) for providing greenhouse space and advice for growing tomatoes for experimental work. We are grateful to Professor Bill Wilson, Dr Tet Woo Lee, and Mary Spellman for facilitating access to, and providing training for, the irradiation facilities at the Auckland Cancer Society Research Centre (Faculty of Medical and Health Sciences, University of Auckland). We also thank Kate Richards (PFR) for providing advice for statistical analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work, representing part of a PhD project, was supported by the University of Auckland Doctoral Scholarship and the New Zealand Plant Protection Society Research Scholarship. The wider project was also assisted by a postgraduate scholarship from the Bragato Research Institute and an international travel grant from Zespri International Limited (BS20139). This study also received support from two projects in the Better Border Biosecurity (B3) research collaboration; ‘Improving risk prediction and reducing uncertainty pre-release for classical biocontrol agents (host testing)’ (P/321021/09) and ‘Socially acceptable eradication tools underpinned by modelling for eradication (BMSB, QFF, Codling moth, SWD)’ (P/321025/05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horrocks, K.J., Avila, G.A., Holwell, G.I. et al. Behaviour and fitness impacts of irradiation-induced sterility in an egg parasitoid and potential implications for their use for insect eradication. J Pest Sci 97, 841–851 (2024). https://doi.org/10.1007/s10340-023-01657-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01657-x