Abstract
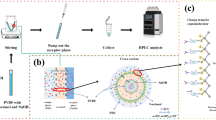
A CE combined with amperometric detection method has been developed for simultaneous determination of nine environmental endocrine disruptors, including bisphenol A, methylparaben, ethylparaben, propylparaben, 4-tert-butylphenol, diethylstilbestrol, 17α-ethinylestradiol, β-estradiol and estriol. In this work, various experimental parameters affecting enrichment factors, electrophoretic separation and detection were investigated in detail. And poly(sodium 4-styrenesulfonate) was selected as the buffer modifier, resulting in considerable improvement of the resolution of the target analytes. Under the optimum conditions, nine analytes could be well separated within 30 min in a 10 g/L poly(sodium 4-styrenesulfonate) modified 60 mmol/L Na2B4O7–H3BO3 buffer (pH 7.60). For each analyte good linear correlation between electrochemical response and its concentration at two or three grades was obtained, the method detection limits reached 0.047–13 ng/mL (S/N = 3) with the assistance of hollow-fiber liquid-phase microextraction, and the highest enrichment factor was up to 255-fold. This proposed method has been applied for the direct analyses of real environmental water and tap water samples without need of derivatization, the average recoveries were in the range of 81.4–117 %, and the corresponding relative standard deviations were lower than 6.2 %.




Similar content being viewed by others
References
Soto AM, Calabro JM, Prechtl N, Yau A, Orlando E, Daxenberger A, Kolok AL, Guillette J, Bizec BL, Lange I, Sonnenschein C (2004) Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in Eastern Nebraska USA. Environ Health Perspect 112:346–352
Brian J, Harris C, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfa A, Marcomini A, Sumpter J (2005) Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect 113:721–728
Adamusova H, Bosakova Z, Coufal P, Pacakova V (2014) Analysis of estrogens and estrogen mimics in edible matrices—a review. J Sep Sci 37:885–905
LaFleur AD, Schug KA (2011) A review of separation methods for the determination of estrogens and plastics-derived estrogen mimics from aqueous systems. Anal Chim Acta 696:6–26
Okada K, Hashimoto S, Imaoka S (2010) Biological functions of protein disulfide isomerase as a target of phenolic endocrine-disrupting chemicals. J Health Sci 56:1–13
Boberg J, Taxvig C, Christiansen S, Hass U (2010) Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 30:301–312
Haman C, Dauchy X, Rosin C, Munoz JF (2015) Occurrence, fate and behavior of parabens in aquatic environments: a review. Water Res 68:1–11
Cabaleiro N, De La Calle I, Bendicho C, Lavilla I (2014) An overview of sample preparation for the determination of parabens in cosmetics. Trends Anal Chem 57:34–46
Kozlowska-Tylingo K, Namiesnik J, Gorecki T (2010) Determination of estrogenic endocrine disruptors in environmental samples: a review of chromatographic methods. Crit Rev Anal Chem 40:194–201
Tijani JO, Fatoba OO, Petrik LF (2013) A review of pharmaceuticals and endocrine-disrupting compounds: sources, effects, removal, and detections. Water Air Soil Pollut 224:1–29
Chen B, Huang Y, He M, Hu B (2013) Hollow fiber liquid–liquid–liquid microextraction combined with high performance liquid chromatography-ultraviolet detection for the determination of various environmental estrogens in environmental and biological samples. J Chromatogr A 130:17–26
Liu M, Qiu B, Jin X, Zhang L, Chen X, Chen G (2008) Determination of estrogens in wastewater using three-phase hollow fiber-mediated liquid-phase microextraction followed by HPLC. J Sep Sci 31:622–628
Diaz-Alvarez M, Turiel E, Martin-Esteban A (2013) Hollow fiber liquid-phase microextraction of parabens from environmental waters. Int J Environ Anal Chem 93:727–738
Tan X, Song Y, Wei R, Yi G (2012) Determination of trace bisphenol-A in water using three-phase hollow fiber liquid phase microextraction coupled with high performance liquid chromatography. Chin J Anal Chem 40:1409–1414
Chao Y, Jian Z, Tu Y, Wang H, Huang Y (2013) An on-line push/pull perfusion-based hollow-fiber liquid-phase microextraction system for high-performance liquid chromatographic determination of alkylphenols in water samples. Analyst 138:3271–3279
Wang X, Qi W, Zhao X, Lu T, Wang X, Zheng L, Yan Y, You J (2014) Determination of four phenolic endocrine disruptors in environmental water samples by high performance liquid chromatography-fluorescence detection using dispersive liquid-liquid microextraction coupled with derivatization. Chin J Chromatogr 32:623–628
Hu Y, Zheng Y, Zhu F, Li G (2007) Sol–gel coated polydimethylsiloxane/β-cyclodextrin as novel stationary phase for stir bar sorptive extraction and its application to analysis of estrogens and bisphenol A. J Chromatogr A 1148:16–22
López-Darias J, Germán-Hernández M, Pino V, Afonso AM (2010) Dispersive liquid–liquid microextraction versus single-drop microextraction for the determination of several endocrine-disrupting phenols from seawaters. Talanta 80:1611–1618
Prichodko A, Jonusaite K, Vickackaite V (2009) Hollow fibre liquid phase microextraction of parabens. Cent Eur J Chem 7:285–290
Basheer C, Lee HK (2004) Analysis of endocrine disrupting alkylphenols, chlorophenols and bisphenol-A using hollow fiber-protected liquid-phase microextraction coupled with injection port-derivatization gas chromatography-mass spectrometry. J Chromatogr A 1057:163–169
Singh B, Kumar A, Malik AK (2014) Recent advances in sample preparation methods for analysis of endocrine disruptors from various matrices. Crit Rev Anal Chem 44:255–269
Piao C, Chen L, Wang Y (2014) A review of the extraction and chromatographic determination methods for the analysis of parabens. J Chromatogr B 969:139–148
Wang H, Zhang A, Wang W, Zhang M, Liu H, Wang X (2013) Separation and determination of triclosan and bisphenol A in water, beverage, and urine samples by dispersive liquid-liquid microextraction combined with capillary zone electrophoresis-UV detection. J AOAC Int 96:459–465
Maijo I, Borrull F, Aguilar C, Calull M (2013) Different strategies for the preconcentration and separation of parabens by capillary electrophoresis. Electrophoresis 34:363–373
Xiao Q, Li Y, Zou X (2009) Determination of phenolic environmental estrogens in food packing materials by nonaqueous capillary electrophoresis –chemiluminescence. Chinese J Anal Chem 37:1611–1616
Dominguez-Alvarez J, Rodriguez-Gonzalo E, Hernandez-Mendez J, Carabias-Martinez R (2012) Programed nebulizing-gas pressure mode for quantitative capillary electrophoresis-mass spectrometry analysis of endocrine disruptors in honey. Electrophoresis 33:2374–2381
Jiang X, Jiang Y, Shi G, Zhou T (2014) Graphene oxide coated capillary for the analysis of endocrine-disrupting chemicals by open-tubular capillary electrochromatography with amperometric detection. J Sep Sci 47:1671–1678
Wu T, Wang WY, Jiang LM, Chu QC, Delaire J, Ye JN (2008) Determination of natural and synthetic endocrine-disrupting chemicals (EDCs) in sewage based on SPE and MEKC with amperometric detection. Chromatographia 68:339–344
Liu S, Wu X, Xie Z, Lin X, Guo L, Yan C, Chen G (2005) On-line coupling of pressurized capillary electrochromatography with end-column amperometric detection for analysis of estrogens. Electrophoresis 26:2342–2350
Mahugo-Santana C, Sosa-Ferrera Z, Torres-Padron ME, Santana-Rodriguez JJ (2011) Application of new approaches to liquid-phase microextraction for the determination of emerging pollutants. Trends Anal Chem 30:731–748
Armenta S, Garrigues S, de la Guardia M (2015) The role of green extraction techniques in green analytical chemistry. Trends Anal Chem 71:2–8
Spietelun A, Marcinkowski L, de la Guardia M, Namieśnik J (2014) Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 119:34–45
Pedersen-Bjergaard S, Rasmussen KE (1999) Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem 71:2650–2656
Tong F, Zhang Y, Chen F, Ma G, Chen Y, Liu K, Dong J, Ye JN, Chu QC (2013) Hollow-fiber liquid-phase microextraction combined with capillary electrophoresis for trace analysis of sulfonamide compounds. J Chromatogr B 942:134–140
Angelov T, Vlasenko A, Tashkov W (2007) HPLC determination of pKa of parabens and investigation on their lipophilicity parameters. J Liq Chromatogr Relat Technol 31:188–197
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 21205042), the Open Funds of the State Key Laboratory of Electroanalytical Chemistry (No. SKLEAC201508), the Daxia Foundation of ECNU (No. 2014DX-077), and the Students Innovative Experimental Project of Shanghai Municipality (No. 201510269070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, F., Zheng, Y., Wang, T. et al. Simultaneous Determination of Phenolic Endocrine Disruptors in Water Samples by Poly(sodium 4-styrenesulfonate) Modified CE Coupled with Hollow-Fiber Liquid-Phase Microextraction. Chromatographia 79, 619–627 (2016). https://doi.org/10.1007/s10337-016-3073-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3073-5