Abstract
Although geolocator tracking of small passerines has become commonplace in the last two decades, this tool is still underutilized for gathering natural history data of migration in many species. Common Whitethroat (Curruca communis) is one of the most numerous Afro-Palearctic migrants. However, our knowledge of the species’ migration ecology remains limited. Here, we combine ring-recovery analyses with geolocator tracking to describe migration routes and population-specific non-breeding areas in this long-distance migrant. Linkages between breeding, passage and non-breeding areas based on ring-recovery records revealed a strong correlation in longitudes, indicating parallel migration system and population-specific non-breeding grounds in whitethroats. Migration route simulation using EURING data suggested a possible migratory divide in Central Europe in autumn, while broad front migration across the Mediterranean occurs in spring. Geolocator-tracked whitethroats from Czechia (Central Europe) and Latvia (North-eastern Europe) migrated to non-breeding sites in Central Africa, where they first resided in the Sahel region before moving farther to a second non-breeding site in November/December. Major stopovers were associated with crossing of ecological barriers. A single Latvian bird with a repeat track showed similar migration patterns in both years, possibly visiting the same non-breeding sites in consecutive years. Both ringing and tracking data revealed clockwise loop migration between breeding and non-breeding sites.
Zusammenfassung
Saisonale Unterschiede der Zugrouten bei Dorngrasmücken (Curruca communis)
Die Dokumentation naturhistorischer Fakten zum Zug kleiner Sperlingsvogelarten mit Hilfe von Geolokation erfährt trotz der Etablierung der Methode in den letzten zwei Jahrzehnten oft noch zu wenig Beachtung. Die Dorngrasmücke (Curruca communis) ist eine der häufigsten Afro-Paläarktischen Zugvogelarten; unser Wissen über ihre Zugökologie ist jedoch nach wie vor begrenzt. In unserer Studie kombinieren wir Ringwiederfundanalyse mit Geolokator-Tracking, um Zugrouten und populationsspezifische Nichtbrutgebiete dieses Langstreckenziehers zu beschreiben. Die Ringwiederfunddaten zeigen eine starke Korrelation zwischen den Längengraden der Brut-, Durchzugs- und Nichtbrutgebiete. Dies deutet auf ein paralleles Nord-Süd-Zugsystem und auf populationsspezifische Nichtbrutgebiete der Dorngrasmücke hin. Ein Simulationsmodel der Zugrouten anhand von EURING-Wiederfunddaten weist auf eine Zugscheide in Europa während des Herbstzuges hin, während der Frühjahrszug in breiter Front über das gesamte Mittelmeer erfolgt. Mit Geolokatoren ausgestattete Dorngrasmücken aus Tschechien (Mitteleuropa) und Lettland (Nordosteuropa) wanderten zu Nichtbrutgebieten in Zentralafrika. Die Vögel hielten sich zunächst in der Sahelzone auf, ehe sie im November/Dezember zu einem zweiten Nichtbrutplatz weiterzogen. Wichtige Zwischenstopps auf dem Zug waren mit der Überquerung ökologischer Barrieren verbunden. Eine lettische Dorngrasmücke, die in zwei aufeinanderfolgenden Jahren verfolgt wurde, zeigte in beiden Jahren ähnliche Zugmuster, wobei sie möglicherweise dieselben Nichtbrutplätze aufsuchte. Sowohl die Beringungs- als auch die Geolokator-Daten belegen, dass Dorngrasmücken zwischen europäischen Brut- und afrikanischen Nichtbrutgebieten im Uhrzeigersinn hin- und herziehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migratory birds take advantage of seasonally occurring resources for their reproduction and survival. To do so, they often travel considerable distances during the annual cycle and, hence, their migration programs, physiology, and morphology have been evolutionary adapted to the spatiotemporal occurrence of resources. However, rapidly changing global environmental conditions have pushed birds to adapt their migration strategies in response. There is strong evidence for such adaptations during the last decades, including shifting migration phenology (Horton et al. 2020), decreasing migration distance (Visser et al. 2009; Rotics et al. 2017), and propensity (Plummer et al. 2015) as well as shifts in breeding and non-breeding distributions (Hill et al. 1998; Curley et al. 2020). For many species and populations, we still lack basic knowledge on their year-round whereabouts and movement patterns. Recording and documenting this information for different species is of high priority for conservation as well as for understanding and predicting the impacts and consequences of climate change on migratory animals in the Anthropocene (Callaghan et al. 2018). Thus, acquiring a proper yardstick—a measure of the current state of things—is a high priority for future records (Visser and Both 2005).
Traditionally, the direction, destination, and timing of bird migration have been studied using individual marking—mainly with unique metal leg rings (Baillie et al. 2007; du Feu et al. 2016). Recovery records of ringed birds have been systematically collected since the beginning of the twentieth century, and they have provided most of the fundamental understanding of where and when different species and populations migrate (e.g. Bairlein 2001; Bairlein et al. 2014). With advanced analytical tools, ring-recovery data can be used to reconstruct population-specific migration routes (Musitelli et al. 2019) and assess migratory connectivity (Cohen et al. 2018) or spatially explicit survival probabilities (Schirmer et al. 2023). Nevertheless, an unavoidable downside of ringing-based studies is the generally low recovery rate of marked individuals in regions such as sub-Saharan Africa, and hence geographic biases in recovery data (Perdeck 1977; Fiedler et al. 2007). For selected species, this has resulted in virtually no recovery records from their non-breeding areas despite more than 100 years of bird ringing. Modern tracking technologies like light-level geolocators (Bächler et al. 2010; Lisovski et al. 2012), despite their limitations, can help partially fill in these knowledge gaps. In particular, our understanding of individual non-breeding sites and migration timing (e.g. van Wijk et al. 2013; Van Bemmelen et al. 2016; Pedersen et al. 2018), as well as links between different breeding and non-breeding populations (i.e. migratory connectivity; Webster et al. 2002; Bauer et al. 2015; Finch et al. 2017), can be improved using tracking technology. Hence, integrating ringing and tracking data can help to elucidate the unknowns in the movement ecology of hard-to-study species.
From the conservation point of view, understanding migratory connectivity can aid in developing appropriate conservation strategies. For example, if individuals from a single breeding population spread across a large non-breeding area, i.e. low migratory connectivity, conservation efforts on a specific area of the non-breeding grounds could benefit a large proportion of individuals from across a large area of the species breeding range. Conversely, if individuals from a single breeding population have low spread and population-specific non-breeding grounds, i.e. high migratory connectivity, conservation efforts on a specific area of the non-breeding grounds would aid only a small proportion of the species’ global breeding population (Webster et al. 2002; Cresswell 2014).
Common Whitethroat (Curruca communis; hereafter, whitethroat) is a long-distance migrant overwintering in the arid and semi-arid zones of sub-Saharan Africa (BirdLife International and Handbook of the Birds of the World 2019). Despite being one of the most common migratory species within the Afro-Palearctic migration system (Hahn et al. 2009), little information is available on its migration routes and non-breeding sites (but see da Prato and da Prato 1983; Fransson 1995). A recent study by Tapia-Harris et al. (2022) provided the first available tracks for the species from its non-breeding sites in Nigeria to breeding sites in Europe and back, revealing the use of multiple non-breeding sites. Here, we combine ring-recovery data with novel migration route simulation technique (Musitelli et al. 2019) and geolocator tracking to describe the migration patterns of whitethroats between Europe and Africa. We focus on describing (1) broad-scale patterns in general migration directions of various European populations, (2) links between breeding and non-breeding populations, (3) individual migration routes in autumn and spring, and (4) individual timing of migration. We further compare results from our tracking study conducted at two localities on the species’ breeding grounds in Europe with a recent tracking study carried out on the species’ non-breeding grounds in Nigeria (Tapia-Harris et al. 2022).
Methods
Ringing data
Ring-recovery data of whitethroats were provided by the EURING Data Bank on 13th October 2023 (du Feu et al. 2016) and by the SAFRING database (https://safring.birdmap.africa) on 28th March 2022. Additionally, 12 recoveries were extracted from Pearson et al. (2014). Upon our request, the EURING dataset was filtered for recoveries less than 20 km from the ringing site (local recoveries accounts for ca. 65% of all records) and finally contained 2803 recovery records of 2658 previously ringed individuals. The SAFRING database contained 95 recovery records of 87 individuals. Local recoveries accounted for 88% of all records, and only two recoveries had information of long-distance movements exceeding 200 km. To link potential breeding and non-breeding sites, we combined ring-recovery information from the above-mentioned sources and checked for records where at least one of the locations was within the species’ non-breeding range in sub-Saharan Africa.
Further, we used the EURING dataset to summarize and simulate migration routes taken by whitethroats within Europe and North Africa following the procedure outlined by Musitelli et al. (2019). This method allows simulations of partial and full migration pathways by connecting ring-recovery records that join at nearby geographic locations (on a given grid), but originate from different individuals. To this end, we used records with a movement distance of > 50 km between consecutive encounters (thereby excluding local movements) of the individual and analysed spring and autumn recoveries separately. Since we aimed at a general description of the species’ migration patterns in northern Africa and Europe, we considered all ring recoveries irrespective of the ringing and recovery year as long as both encounters were within the defined spring or autumn migration season. For the autumn migration simulation, we used ring-recovery records where both ringing and recovery occurred between June and December. For spring migration, we used records from a period between January and July. We intentionally included the breeding season in both specified periods as ring-recovery records where one of the nodes is located at the breeding grounds can indicate the migration pathways taken by the individual from and to its breeding site. In each season, we used only movements in the expected migration direction, i.e. northward in spring and southward in autumn, setting a minimum movement threshold between consecutive encounters to 0.2° across latitude. The resulting ring-recovery records used for route simulation amounted to 898 (538 of those were within the same year) for autumn migration and 421 (104 within the same year) for spring migration.
Analyses were done across one-degree grid cells and in cells with divergent migration directions (> 30-degree deviance in migration direction from a minimum of three ring recoveries). Eastward or westward movements were assigned randomly during the simulation procedure. Similarly, starting points for the route simulation were set at random. In spring, the simulation was run backward starting from the breeding grounds, as no information is available on the distribution and density of individuals at the non-breeding areas or on spring passage areas in North Africa (see Musitelli et al. 2019 for details).
Geolocator tracking
Whitethroats were tagged with light-level geolocators at two breeding locations in Czechia and Latvia between 2017 and 2020. In both study sites, birds were captured using mist nets. At the Central European site in Czechia (50.30° N, 16.44 °E), 23 and 31 individuals were tagged during the breeding seasons of 2018 and 2019, respectively, using the GDL-2.1 geolocators (Swiss Ornithological Institute). This included 23 males, 12 females, and 19 unsexed individuals.
At the northeastern European site in Latvia (56.24° N, 25.37° E), 20 individuals were tagged with Intigeo-P50Z11-7-DIP geolocators (Migrate Technology Ltd.) in late July–early August 2017, after the core breeding period of the species. The late deployment was unplanned and resulted from a delay in geolocator delivery. As a result, 4 tags were deployed on juvenile birds, 10 on females, 2 on males, and 4 on unsexed individuals. In each of the 2019 and 2020 breeding seasons, an additional 20 individuals—14 males and 6 females in both years—were tagged using GDL-2.1 geolocators (Swiss Ornithological Institute) between May and August. In all cases, geolocators including the harnesses weighed approximately 0.6 g, representing ca. 4% of the body mass of the tagged birds (mean: 14.4 ± 0.9 g).
In Czechia, we retrieved five geolocators from males (three in 2020 and two in 2021), one of which stopped recording before spring migration. In Latvia, we recovered two geolocators—one from a female in 2018 and one from a male in 2021, 2 years after the deployment. The latter geolocator contained information on 1.5 migration cycles, providing repeated autumn tracks and information on non-breeding sites in two consecutive years.
To obtain location estimates of whitethroats across their annual cycle, light intensity data collected by the geolocators were processed following the guidelines set by Lisovski et al. (2020). We first defined twilights using the R-package TwGeos (Lisovski et al. 2016) with a light-level threshold of one (log-scale), deleting twilights within 14 days of the equinoxes and false twilights created by shading. We obtained reference solar zenith angles from either in-habitat calibration based on light readings at the breeding location or Hill–Ekstrom calibration using light readings at the non-breeding site, depending on the quality and noise of light intensity data at each site. Stark changes in consecutive times of sunrise, sunset, noon, and midnight were used to distinguish movement and stationary periods (defined as stops greater than 2 days).
Next, following the Group model in the R package SGAT, we “grouped” similar twilights into single locations (stationary sites). Based on the twilight error distribution defined during calibration, flight speed distribution (gamma distributed; shape = 2.2, rate = 0.06), and a land mask, which limits stopovers to locations on land, SGAT estimates the most probable locations of the bird using a Bayesian model. We initiated the model by first running a “modifiedGamma” model with relaxed assumptions for 1000 iterations before tuning the model with final assumptions/priors for five runs with 300 iterations. Finally, we ran the model with 2000 iterations, producing the most likely tracks (median location estimates) and their associated 95% probability distributions.
Further, we identified Sahara crossing flights and their timing by manually inspecting daily light patterns and looking for abnormally long periods of the full (or increased) light pattern (see Adamík et al. 2016). We defined migration speed as the rate of movement over a complete migration period, including stopovers. Data analyses were done in R v.4.1.3 (R Core Team 2022).
Results
Ringing data
There were 43 ring recoveries that linked non-breeding grounds in sub-Saharan Africa with passage and breeding areas further north (Fig. 1). These indicated broad-scale longitudinal segregation between ringing and recovery sites, i.e. birds overwintering in West Africa originated from the western part of the breeding range, Central African overwintering sites were occupied by birds from central and northern Europe, and birds found in the eastern part of the non-breeding range originated from the eastern part of the breeding range (correlation between the ringing and recovery longitudes: r(41) = 0.935, p < 0.001).
Ring recoveries of Common Whitethroats (Curruca communis) linking non-breeding areas (light grey background) with breeding grounds (dark grey background) and passage areas in between. Black great circle lines indicate individual connections between ringing and recovery events, and the colour of the dots designates the timing of these events—a monthly legend is given in the colour wheel
In autumn, migration route simulation suggested a potential migratory divide between the Western and the Eastern flyways in Central Europe at around 10° E (Fig. 2). In contrast, during spring, simulated routes showed a broad-front migration across Europe and the Mediterranean, with many of the modelled routes crossing the Mediterranean Sea either directly or via the Balearic and Italian islands, and Corsica (i.e. Central Mediterranean flyway) rather than circumventing via the Iberian Peninsula or the Middle East. As a general pattern, the simulated migration routes in spring were found more to the west than in autumn (mean longitude of routes: autumn = 12.2° E ± 13.2 (SD), spring = 8.7° E ± 11.3, t-test: t = − 4.502, df = 998, p < 0.001), revealing a clockwise loop migration pattern in whitethroats.
Geolocator tracking
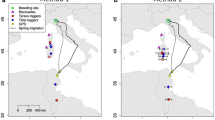
All seven geolocator-tracked individuals from Czechia and Latvia migrated to non-breeding sites in Central Africa and resided in the broader surroundings of Lake Chad between 6° and 15° E (Fig. 3). While in sub-Saharan Africa, all but one bird with available data utilized two distinct non-breeding sites, moving between them in mid-November and December. On average, these sites were located 320 ± 120 km (SD) from one another. The final and main non-breeding sites of the two Latvian birds were located more southward (N Nigeria) than the five Czech birds (S Niger, N Cameroon, and W Chad; Fig. 3). The average great circle distance between the breeding and main non-breeding residency sites was 3890 ± 120 km for the Czech birds and 5020 ± 40 km for the Latvian birds. For both populations, autumn migration routes traversed the Balkan Peninsula and tracked whitethroats crossed the Mediterranean between 15° and 25° E before arriving at the non-breeding sites. In spring, migration routes were located west of the autumn routes traversing Sardinia, Corsica, Sicily, and the Apennine Peninsula between 5° and 15° E. Thus, the annual migration tracks of whitethroats conformed to a clockwise loop migration pattern.
Geolocator tracks of seven Common Whitethroats (Curruca communis) breeding in Czechia (CZ) and Latvia (LV). Median stationary location estimates (dots and squares) are shown alongside their interquartile ranges (grey bars). Panels LV-2.1 and LV-2.2 show the same individual that was tracked for 1.5 years, thus providing information on two consecutive autumn migrations and non-breeding sites. Lower panel shows zoomed-in area of all non-breeding sites of the tracked individuals (light blue—Czech birds, dark blue—Latvian birds)
Autumn migration started in late August and early September (mean ± SD; CZ: 25-Aug ± 4 days; LV: 29-Aug ± 5 days), and birds arrived at their respective non-breeding sites in the second half of September and early October (CZ: 20-Sep ± 9 days; LV: 26-Sep ± 7 days; Fig. 4). Major stopovers were made before crossing ecological barriers—the Mediterranean Sea and the Sahara Desert.
Individual migration schedules of seven Common Whitethroats (Curruca communis) tracked with geolocators. Red—breeding site, orange—autumn migration, blue—non-breeding period with the timing of site change indicated in light gray, green—spring migration, dark grey—Sahara crossing; colour tone designates the different breeding origin of the individuals: darker—Latvia, lighter—Czechia. IDs LV-2 and LV-2.1 refer to the same individual which carried the logger for two consecutive years. Incomplete lines indicate logger stoppage
In spring, two migration strategies emerged: (1) the two Latvian and one out of four Czech birds initially moved west and made a ca. 4–5 weeks long stopover in the Sahel before crossing the Sahara Desert, while (2) the other three Czech birds with available spring tracks did not make prolonged stopovers before Sahara crossing. Further, most tracked birds stopped over after the Sahara crossing before they arrived at their respective breeding sites.
The three birds which had lengthy spring stopovers in the Sahel region spent on average 184 ± 8 days (SD) at their non-breeding residency sites, while the three birds without the prolonged stopovers spent 212 ± 6 days (SD) at their non-breeding sites. This 4-week difference mainly resulted from the earlier spring departure of the former group (mean ± SD: 29-Mar ± 2 days vs 16-Apr ± 7 days), which also had considerably longer spring migration compared to birds that did not make a prolonged stopover in the Sahel (47 ± 2 days vs 18 ± 2 days). The only Czech bird making a stopover in the Sahel arrived at the breeding site at a similar time to the two Latvian birds, but ca. 2 weeks later than the mean arrival of the three other Czech birds (17-May vs 4-May ± 6 days).
Migration speed in autumn varied considerably between individuals, averaging at 164 ± 34 km/day (range 99–207 km/day). Migration speed in spring was more than twofold faster for individuals which did not make the prolonged stopover in Sahel (225 ± 15 km/day) as compared to the individuals that did (99 ± 19 km/day).
Across all tracked individuals, the mean duration of each of the four main annual cycle parts was as follows: autumn migration = 27 ± 6 days (7% of the annual cycle), non-breeding residency = 198 ± 16 days (54%), spring migration = 32 ± 16 days (9%), and breeding site residency = 108 ± 5 days (30%; Fig. 4).
The single Latvian bird with a repeat autumn track showed similar migration patterns in both years with a 2–3 weeks long stopover at the Balkan Peninsula and a non-breeding site in the same region in north-eastern Nigeria (Fig. 3). The distance between the mean estimates of the two non-breeding sites was 220 km, but 95% CIs of the site estimates were overlapping and, thus, it cannot be statistically excluded that the bird visited the same non-breeding site in two consecutive years. The start of autumn migration in the two years differed by only four days, while the timing of the Sahara crossing and arrival at the non-breeding residency site(s) differed by 12 and 15 days, respectively (Fig. 4).
Discussion
Our results suggest relatively strong longitudinal separation in the global population of whitethroats and provide evidence for the existence of clockwise loop migration, at least for Central, Northern, and Eastern European populations. Moreover, migration route simulations based on ring recoveries suggest a migratory divide in Central Europe in autumn—a pattern characteristic for many species in this region (Cepák et al. 2008). These results are further supported by our geolocator tracking, as autumn migration routes of individuals from both tracked populations converged over the Balkan Peninsula. Our results of geolocator tracking largely coincide with tracking data gathered on the species’ non-breeding grounds in Nigeria (Tapia-Harris et al. 2022), with both studies linking breeding sites in Central and Eastern Europe with non-breeding sites in Lake Chad area.
Ring recoveries and geolocator tracks alike suggest more westerly migration routes in spring compared to autumn. These findings are also supported by observational data in, for example, Morocco, where whitethroats are widespread in spring, but uncommon in autumn (Thévenot et al. 2003). Such loop migration has typically emerged as an adaptation to seasonally uneven distribution of resources, e.g. food availability at stopovers and atmospheric conditions during flight bouts (La Sorte and Fink 2017; Vansteelant et al. 2017). A contributing factor to the observed pattern might be the later onset of spring in Southeast Europe and the Balkan Peninsula than in southwest Europe, central Mediterranean islands, and the Apennine Peninsula (Briedis et al. 2024) where food availability peaks earlier in the season, enabling feeding opportunities for migrants. A modelling attempt by Kranstauber et al. (2015) also found that optimal routes in respect to wind regime were located more westward in spring compared to autumn for migratory birds travelling between Europe and sub-Saharan Africa. Our results on the migration pattern of whitethroats are in line with these findings, suggesting that birds following the clockwise loop migration pattern might benefit from favourable atmospheric conditions in both seasons. However, atmospheric conditions and the distribution of favourable winds are predicted to change considerably in the future due to climate change (Arias et al. 2021). This might pose risks to the long-established migratory programmes of whitethroats and other species following similar migration routes.
One of the main conclusions in an earlier study by Tapia-Harris et al. (2022) was that whitethroats exhibit low migratory connectivity. Birds were tracked from a single location on the non-breeding ground in Nigeria and migrated to breeding sites spread between Central Europe and western Russia, between 48°–58° N and 18°–33° E, suggesting population mixing in sub-Saharan Africa. However, the geographic scale, the number of sampled populations, and distances between them are fundamental aspects when evaluating migratory connectivity (Cohen et al. 2018). Due to the differences in data availability and its spatial coverage, ring recoveries on the non-breeding grounds (Fig. 1) and geolocator tracks (Fig. 3) in our study suggest contrasting patterns of migratory connectivity when scrutinized separately. The pronounced longitudinal segregation in ring-recovery records between breeding and non-breeding regions implies population-specific non-breeding areas and, thus, strong migratory connectivity (Webster et al. 2002). On the contrary, longitude of the non-breeding sites largely overlap between geolocator-tracked individuals from Latvia and Czechia, implying population mixing on the non-breeding grounds and, thus, low migratory connectivity (Finch et al. 2017) for populations breeding in Central and Northeastern Europe. Tracking studies within the Afro-Palearctic migratory network are often biased towards (western) Europe (see Briedis et al. 2020), while for many long-distance migrants, breeding distributions often stretch further east to the Ural Mountains and beyond, where a large proportion of the global population breeds (Keller et al. 2020). As a result, only a subpart of the global breeding range is often sampled (tracked) when evaluating migratory connectivity (e.g. see Adamík et al. 2023). Our example of comparing ring-recovery records with tracking data illustrates the risk for misinterpretation when assessing migratory connectivity if only a part of the global distribution is sampled. This may be particularly important when quantifying species-specific strength of migratory connectivity (e.g. in a comparative framework; Finch et al. 2017) as such quantitative estimates are highly sample sensitive.
Interestingly, most tracked birds visited two non-breeding sites, moving between them in November–December. These results match the findings of Tapia-Harris et al. (2022), who tracked six whitethroats from their non-breeding sites in central Nigeria and found that birds utilized two non-breeding sites. First, a more northernly located site in the Sahel region and a second, more southern site in central Nigeria, moving between them in November–early December. Thus, non-breeding site itinerancy seems to be a common overwintering strategy, for whitethroats migrating to central Africa, but also for another congeneric species, the Barred Warbler migrating to Eastern Africa (Wong et al. 2024). Overall, there is an accumulating number of tracking studies that have described similar itinerancy patterns in a suite of species that migrate to the Sahel region (Briedis et al. 2016a; Thorup et al. 2017; Koleček et al. 2018; Wong et al. 2022). This phenomenon was already described by Moreau (1972), and the decrease in resource availability in the Sahel region following the rainy season is thought to be the main driver (Zwarts et al. 2009).
While ring-recovery data provide only fragmentary information on individual migration timing, geolocator tracking can deliver full annual schedules of the tracked birds. This includes timing of critical events—departure and arrival at the breeding and main non-breeding sites—and derived information like migration duration and speed (Briedis et al. 2019, 2020). As expected from geographic patterns in long-distance migration timing (Conklin et al. 2010; Briedis et al. 2016b, 2020), more northernly breeding Latvian birds lagged behind the Czech whitethroats at all key migration events. The peculiar differences in spring migration tactics, with some birds making a prolonged stopover before Sahara crossing and others not, may be related to deteriorating conditions at the non-breeding sites as migration approaches—the so-called Moreau’s paradox (Schlaich et al. 2016). As the dry season in the Sahel progresses in March and April, long-distance migrants breeding in Europe need to prepare and fuel for the cross-desert migration to their breeding sites (Moreau 1972). It may be that the difference in the two strategies found in whitethroats showcases a high degree of phenotypic plasticity in the species regarding spring movement strategies. Individuals residing at non-breeding sites where conditions become increasingly unfavourable may flexibly leave these sites and fuel for migration elsewhere. At the same time, individuals residing at non-breeding sites with plentiful resources may fuel for the first leg of migration—the cross-Sahara flight—directly at their non-breeding sites. As a result, they spend approximately 60% of the annual cycle at a single site in the Sahel, highlighting the importance of the Sahel region for whitethroats (Zwarts et al. 2009; Tapia-Harris et al. 2022).
Every autumn, roughly 80 million whitethroats migrate from the breeding areas in Europe to their non-breeding regions in sub-Saharan Africa (Hahn et al. 2009). Yet, century-long bird ringing efforts have resulted in a mere 43 ring-recovery records from the species’ non-breeding areas. While tracking studies are typically of small sample size and temporal coverage (often one or only a few years), limiting the strength of conclusions that can be drawn from them, supplementing tracking data with other data types, e.g. ring recoveries and stable isotopes (Boulèt et al. 2006; Lisovski et al. 2019), can provide detailed insights into the ecology of little-known and hard-to-study species. Gathering and publishing such basic information on species' natural histories are becoming increasingly rare in modern ornithological science (Callaghan et al. 2018). Yet, such knowledge remains a cornerstone for conservation, management, and future record in the light of global change.
Data availability
Tracking data is stored on MoveBank (study IDs 4310298483 and 4310399048), and raw geolocator data along with data analyses files are deposited on Zenodo open data repository (https://doi.org/10.5281/zenodo.13311912).
References
Adamík P, Emmenegger T, Briedis M et al (2016) Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Sci Rep 6:21560. https://doi.org/10.1038/srep21560
Adamík P, Bureš S, Hahn S et al (2023) Timing of migration and African non-breeding grounds of geolocator-tracked European Pied Flycatchers: a multi-population assessment. J Ornithol 164:875–886. https://doi.org/10.1007/s10336-023-02081-9
Arias PA, Bellouin N, Coppola E et al (2021) Technical summary. In: Masson-Delmotte V, Zhai P, Pirani A et al (eds) Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 33–144
Bächler E, Hahn S, Schaub M et al (2010) Year-round tracking of small trans-Saharan migrants using light-level geolocators. PLoS ONE 5:e9566. https://doi.org/10.1371/journal.pone.0009566
Baillie S, Bairlein F, Clark J et al (2007) Bird ringing for science and conservation
Bairlein F (2001) Results of bird ringing in the study of migration routes. Ardea 89:7–19
Bairlein F, Dierschke J, Dierschke V et al (2014) Atlas des Vogelzugs. Ringfunde deutscher Brut- und Gastvögel. AULA-Verlag, Wiebelsheim
Bauer S, Lisovski S, Hahn S (2015) Timing is crucial for consequences of migratory connectivity. Oikos 125:605–612. https://doi.org/10.1111/oik.02706
BirdLife International and Handbook of the Birds of the World (2019) Bird species distribution maps of the world. Version 2019.1. http://datazone.birdlife.org/species/requestdis
Boulèt M, Gibbs HL, Hobson KA (2006) Integrated analysis of genetic, stable isotope, and banding data reveal migratory connectivity and flyways in the northern Yellow Warbler (Dendroica petechia; aestiva group). Ornithol Monogr 61:29–78. https://doi.org/10.1642/0078-6594(2006)61[29:IAOGSI]2.0.CO;2
Briedis M, Beran V, Hahn S, Adamík P (2016a) Annual cycle and migration strategies of a habitat specialist, the Tawny Pipit Anthus campestris, revealed by geolocators. J Ornithol 157:619–626. https://doi.org/10.1007/s10336-015-1313-3
Briedis M, Hahn S, Gustafsson L et al (2016b) Breeding latitude leads to different temporal but not spatial organization of the annual cycle in a long-distance migrant. J Avian Biol 47:743–748. https://doi.org/10.1111/jav.01002
Briedis M, Bauer S, Adamík P et al (2019) A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc R Soc B 286:20182821. https://doi.org/10.1098/rspb.2018.2821
Briedis M, Bauer S, Adamík P et al (2020) Broad-scale patterns of the Afro-Palaearctic landbird migration. Glob Ecol Biogeogr 29:722–735. https://doi.org/10.1111/geb.13063
Briedis M, Hahn S, Bauer S (2024) Duration and variability of spring green-up mediate population consequences of climate change. Ecol Lett 27:e14380. https://doi.org/10.1111/ele.14380
Callaghan CT, Martin JM, Kingsford RT, Brooks DM (2018) Unnatural history: is a paradigm shift of natural history in 21st century ornithology needed? Ibis 160:475–480. https://doi.org/10.1111/ibi.12555
Cepák J, Klvaňa P, Škopek L et al (2008) Atlas migrace ptáku Ceské a Slovenské Republiky. Aventinum, Prague
Cohen EB, Hostetler JA, Hallworth MT et al (2018) Quantifying the strength of migratory connectivity. Methods Ecol Evol 9:513–524. https://doi.org/10.1111/2041-210X.12916
Conklin JR, Battley PF, Potter MA, Fox JW (2010) Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1:67. https://doi.org/10.1038/ncomms1072
Cresswell W (2014) Migratory connectivity of Palaearctic-African migratory birds and their responses to environmental change: the serial residency hypothesis. Ibis 156:493–510
Curley SR, Manne LL, Veit RR (2020) Differential winter and breeding range shifts: implications for avian migration distances. Divers Distrib 26:415–425. https://doi.org/10.1111/ddi.13036
da Prato SRD, da Prato ES (1983) Movements of Whitethroats Sylvia communis ringed in the British Isles. Ringing Migr 4:193–210. https://doi.org/10.1080/03078698.1983.9673808
du Feu CR, Clark JA, Schaub M et al (2016) The EURING Data Bank – a critical tool for continental-scale studies of marked birds. Ringing Migr 31:1–18. https://doi.org/10.1080/03078698.2016.1195205
Fiedler W, Bairlein F, Köppen U (2007) Using large-scale data from ringed birds for the investigation of effects of climate change on migrating birds: pitfalls and prospects. Adv Ecol Res 35:49–67
Finch T, Butler S, Franco A, Cresswell W (2017) Low migratory connectivity is common in long-distance migrant birds. J Anim Ecol 38:42–49. https://doi.org/10.1111/1365-2656.12635
Fransson T (1995) Timing and speed of migration in North and West European populations of Sylvia Warblers. J Avian Biol 26:39–48. https://doi.org/10.2307/3677211
Hahn S, Bauer S, Liechti F (2009) The natural link between Europe and Africa – 2.1 billion birds on migration. Oikos 118:624–626. https://doi.org/10.1111/j.1600-0706.2008.17309.x
Hill GE, Sargent RR, Sargent MB (1998) Recent change in the winter distribution of Rufous Hummingbirds. Auk 115:240–245. https://doi.org/10.2307/4089135
Horton KG, La Sorte FA, Sheldon D et al (2020) Phenology of nocturnal avian migration has shifted at the continental scale. Nat Clim Chang 10:63–68
Keller V, Herrando S, Voříšek P et al (2020) European breeding bird atlas 2: distribution, abundance and change. European Bird Census Council & Lynx Edicions, Barcelona
Koleček J, Hahn S, Emmenegger T, Procházka P (2018) Intra-tropical movements as a beneficial strategy for Palearctic migratory birds. R Soc Open Sci 5:171675. https://doi.org/10.1098/rsos.171675
Kranstauber B, Weinzierl R, Wikelski M, Safi K (2015) Global aerial flyways allow efficient travelling. Ecol Lett 18:1338–1345. https://doi.org/10.1111/ele.12528
La Sorte FA, Fink D (2017) Migration distance, ecological barriers and en-route variation in the migratory behaviour of terrestrial bird populations. Glob Ecol Biogeogr 26:216–227. https://doi.org/10.1111/geb.12534
Lisovski S, Hewson CM, Klaassen RHGG et al (2012) Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol Evol 3:603–612. https://doi.org/10.1111/j.2041-210X.2012.00185.x
Lisovski S, Sumner MD, Wotherspoon SJ (2016) TwGeos: basic data processing for light based geolocation archival tags. Github
Lisovski S, Németh Z, Wingfield JC et al (2019) Migration pattern of Gambel’s White-crowned Sparrow along the Pacific Flyway. J Ornithol 160:1097–1107. https://doi.org/10.1007/s10336-019-01685-4
Lisovski S, Bauer S, Briedis M et al (2020) Light-level geolocator analyses: a user’s guide. J Anim Ecol 89:221–236. https://doi.org/10.1111/1365-2656.13036
Moreau RE (1972) The Palearctic African bird migration systems. Academic Press, London
Musitelli F, Spina F, Møller AP et al (2019) Representing migration routes from re-encounter data: a new method applied to ring recoveries of Barn Swallows (Hirundo rustica) in Europe. J Ornithol 160:249–264. https://doi.org/10.1007/s10336-018-1612-6
Pearson D, Backhurst G, Jackson C (2014) The study and ringing of Palaearctic birds at Ngulia Lodge, Tsavo West National Park, Kenya, 1969–2012: an overview and update. Scopus 33:1–80
Pedersen L, Jackson K, Thorup K, Tøttrup AP (2018) Full-year tracking suggests endogenous control of migration timing in a long-distance migratory songbird. Behav Ecol Sociobiol 72:139. https://doi.org/10.1007/s00265-018-2553-z
Perdeck AC (1977) The analysis of ringing data: pitfalls and prospects. Vogelwarte 29:33–44
Plummer KE, Siriwardena GM, Conway GJ et al (2015) Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Glob Chang Biol 21:4353–4363. https://doi.org/10.1111/gcb.13070
R Core Team (2022) R: a language and environment for statistical computing
Rotics S, Turjeman S, Kaatz M et al (2017) Wintering in Europe instead of Africa enhances juvenile survival in a long-distance migrant. Anim Behav 126:79–88. https://doi.org/10.1016/j.anbehav.2017.01.016
Schirmer S, Korner-Nievergelt F, Von Rönn JAC, Liebscher V (2023) Estimating survival in continuous space from mark-dead-recovery data-towards a continuous version of the multinomial dead recovery model. J Theor Biol 574:22–5193. https://doi.org/10.5281/zenodo.6
Schlaich AE, Klaassen RHG, Bouten W et al (2016) How individual Montagu’s Harriers cope with Moreau’s Paradox during the Sahelian winter. J Anim Ecol 85:1491–1501. https://doi.org/10.1111/1365-2656.12583
Tapia-Harris C, Izang A, Cresswell W (2022) Migratory routes, breeding locations and multiple non-breeding sites of Common Whitethroats Curruca communis revealed by geolocators. PLoS ONE 17:1–20. https://doi.org/10.1371/journal.pone.0274017
Thévenot M, Bergier P, Vernon R (2003) The birds of Morocco. BOU & BOC, Herts
Thorup K, Tøttrup AP, Willemoes M et al (2017) Resource tracking within and across continents in long-distance bird migrants. Sci Adv 3:E1601360. https://doi.org/10.1126/sciadv.1601360
van Bemmelen RSA, Hungar J, Tulp I, Klaassen RHG (2016) First geolocator tracks of Swedish Red-necked Phalaropes reveal the Scandinavia-Arabian Sea connection. J Avian Biol 47:295–303. https://doi.org/10.1111/jav.00807
van Wijk RE, Schaub M, Tolkmitt D et al (2013) Short-distance migration of Wrynecks Jynx torquilla from Central European populations. Ibis 155:886–890. https://doi.org/10.1111/ibi.12083
Vansteelant WMG, Shamoun-Baranes J, van Manen W et al (2017) Seasonal detours by soaring migrants shaped by wind regimes along the East Atlantic Flyway. J Anim Ecol 86:179–191. https://doi.org/10.1111/1365-2656.12593
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc Roy Soc B 272:2561–2569. https://doi.org/10.1098/rspb.2005.3356
Visser ME, Perdeck AC, van Balen JH, Both C (2009) Climate change leads to decreasing bird migration distances. Glob Chang Biol 15:1859–1865. https://doi.org/10.1111/j.1365-2486.2009.01865.x
Webster MS, Marra PP, Haig SM et al (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83. https://doi.org/10.1016/S0169-5347(01)02380-1
Wong JB, Turon F, Fernández-Tizón M, Hahn S (2022) First insights into migration routes and nonbreeding sites used by Red-rumped Swallows (Cecropis daurica rufula) breeding in the Iberian Peninsula. J Ornithol 163:1045–1049. https://doi.org/10.1007/s10336-022-02011-1
Wong JB, Adamík P, Bažant M, Hahn S (2024) Migration and daily flight activity patterns in the Barred Warbler Curruca nisoria over the annual cycle. J Vertebr Biol 73:23085. https://doi.org/10.25225/jvb.23085
Zwarts L, Bijlsma R, van der Kamp J, Wymenga E (2009) Living on the edge: wetlands and birds in a changing Sahel. KNNV Publishing, Zeist
Acknowledgements
We are thankful to all bird ringers for their dedicated work over the years in gathering ring-recovery data and Dorian Moss at the EURING Data Bank, and Kim Hunt at the SAFRING for kindly helping with our data requests. Aline Knoblauch kindly provided the Common Whitethroat illustration in Figure 3. This is publication #6 of the Tracking Least Known Species project of the Swiss Ornithological Institute.
Funding
Open access funding provided by Swiss Ornithological Institute. MB received support from the Latvian Council of Sciences (Grant award number: lzp-2023/1-0233). PA received support from the Czech Science Foundation (project 20-00648S).
Author information
Authors and Affiliations
Contributions
MB and SH designed the study. TL and SH provided geolocators. PA, KF, MK, KH, and JP organized and conducted fieldwork. MB requested and processed the ring-recovery data. JBW analysed the geolocator data and wrote parts of methods. MB wrote the original draft of the manuscript. All authors reviewed, commented on, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
None.
Ethics approval
Deployment of geolocators was granted by ringing licences issued by the National Museum in Prague and the Nature Conservation Agency of Latvia.
Additional information
Communicated by N. Chernetsov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Briedis, M., Wong, J.B., Adamík, P. et al. Seasonal variation in migration routes of Common Whitethroat Curruca communis. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02204-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02204-w