Abstract
We tested whether microhabitat use affects dispersal and population differentiation in forest birds of the southwestern Palaearctic, a link previously suggested in Neotropical birds. To approach this, the number of subspecies within 32 species was used as a metric of population differentiation and was related to their feeding substrata and seasonal changes in abundance (a surrogate of dispersal) in a mountain range (Guadarrama Mountains, Central Spain). Multivariate analyses in which the effect of range size (a main correlate of within-species diversification) and phylogeny relatedness were considered, showed that those birds adapted to exploit the tree canopy had lower seasonal changes in abundance and more subspecies than ground-dweller birds. Our results support a cause-effect link between the use of stable resources in the canopy, seasonal movements and population differentiation of birds from temperate forests of the southwestern Palaearctic.
Zusammenfassung
Beeinflusst die Nutzung von Mikrohabitaten die Populationsdifferenzierung? Ein Test mit südwest-paläarktischen Waldvögeln.
Wir haben untersucht, ob die Nutzung von Mikrohabitaten die Ausbreitung und Artenbildung bei Waldvögeln der südwestlichen Paläarktis beeinflusst, ein Zusammenhang, der bereits bei neotropischen Vögeln vermutet wurde. Zu diesem Zweck wurde die Anzahl der Unterarten innerhalb von 32 Arten mit ihren Nahrungssubstraten und den saisonalen Veränderungen der Abundanz (stellvertretend für die Ausbreitung) in einem Gebirge (Sierra de Guadarrama, Zentralspanien) in Beziehung gesetzt. Multivariate Analysen, bei denen die Auswirkung der Größe des Verbreitungsgebietes (ein Hauptkorrelat der Diversifizierung innerhalb der Arten) und die phylogenetische Verwandtschaft berücksichtigt wurden, ergaben, dass die Vögel, die an die Nutzung der Baumkronen angepasst sind, geringere saisonale Veränderungen in der Abundanz und mehr Unterarten aufwiesen als bodenbewohnende Vögel. Unsere Ergebnisse belegen einen Ursache-Wirkungs-Zusammenhang zwischen der Nutzung stabiler Ressourcen im Kronendach, saisonalen Bewegungen und der Populationsdifferenzierung von Vögeln der gemäßigten Wälder der südwestlichen Paläarktis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms behind geographical differentiation of populations have been traditionally expressed as the result of the main but not mutually exclusive processes of long-distance dispersal and vicariance (Coyne and Orr 2004). In this context, dispersal ability has been considered a main driver of evolution since it regulates the intensity of gene flow and the rate of differentiation of populations inhabiting a given geographical setting (Slatkin 1987; Kisel and Barraclough 2010). This effect has also been suggested in birds as the less dispersive species show the highest rates of within-species differentiation (Belliure et al. 2000; Claramunt et al. 2011). Thus, it is commonly agreed that dispersal capacity is a key process of the taxonomic diversification in birds (Schweizer and Liu 2018). Dispersal is nevertheless the outcome of a set of processes that must be properly understood to achieve an integrative view of taxonomic diversification (Ronce 2007). It has been suggested, for instance, that seasonal habitats select for greater dispersal ability, whereas stable habitats select for reduced dispersal and increased allopatric speciation (Jocque et al. 2010). In this context, population differentiation in Neotropical forest birds has been related to habitat use as ground-dwellers disperse less than canopy dwellers, because feed on the stable resources of forest floor (Phillimore et al. 2006; Burney and Brumfield 2009; Salisbury et al. 2012; Smith et al. 2014; Miller et al. 2021). However, despite the interest of these ecological links to unravel the processes involved in bird diversification, there are not studies designed to explore these patterns outside the Neotropics.
Here, we study whether population differentiation of 32 southwestern Palaearctic forest birds, as reported by the number of subspecies (Belliure et al. 2000; Phillimore et al. 2007; Martin and Tewksbury 2008; Salisbury et al. 2012), is related to their ability to cope with seasonal changes in food availability. Movements related to seasonal food tracking can erode the reproductive isolation of populations. Thus, it can be predicted that birds reluctant to make these displacements will disperse less and will show higher population differentiation as reported by the number of subspecies than more vagrant birds. We have opted for this taxonomical approach (Phillimore et al. 2006) since the available molecular data on which to perform a similar analysis are still scarce (Pârâu and Wink 2021) and do not cover the full range of population differentiation (Winker 2021; see Methods). We will test this prediction in partial migratory bird species (comprising both migratory and resident individuals; Chapman et al. 2011), which move within the same geographical setting (Western Palaearctic) and have been affected in the past by the same environmental and geographical drivers of genetic differentiation (e.g. glacial processes; Hewitt 2004, 2011). In this context, it has been suggested that canopy birds are more resilient to seasonal changes because food related to barks, tree lichens and foliage does not collapse by winter snowfalls like in the case of ground-dweller birds (Carrascal et al. 1987). If this occurs, canopy birds will show a reduced seasonal change in abundance related to winter dispersal in search of suitable wintering grounds and will show more subspecies than ground-dweller birds, more compelled to leave breeding areas in winter due to the periodic collapse of food.
Methods
Study area
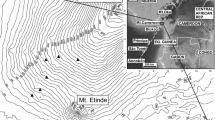
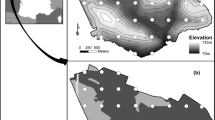
This study was carried out in the Guadarrama Mountains (Spain), a mountain area within the Iberian Plateau that ranges from 600 to 2400 m.a.s.l. (the highest peak at 40.85ºN, – 3.96ºW reaches 2428 m.a.s.l.; Fig S1). These mountains are covered by an altitudinal succession of sclerophyllous Holm Oaks (Quercus ilex), less drought-tolerant Pyrenean Oaks (Quercus pyrenaica) and endemic Scots Pines (Pinus sylvestris var. iberica) in the highest areas, replicating the latitudinal distribution of forest types in the Western Palaearctic (European Environment Agency 2011). These mountains show decreasing temperature and increasing precipitation with elevation that, in winter, produce persistent snow-covered areas at higher elevations. As a result, these highlands suffer from a widespread loss of bird numbers and species that have moved to wintering grounds in southern sectors (Tellería et al. 2001; Carrascal and Palomino 2012). As seasonal change in bird abundance within this elevation gradient replicates the latitudinal rearrangement of bird numbers in the Palaearctic (Busse 2001), we assume that the study area is suitable for the exploration of forest birds' ability to cope with seasonal changes in the environmental conditions of the Western Palaearctic.
Seasonal changes
During the winter of 2018–19 (December-February), we counted birds once in 115 circular sampling points randomly distributed from 700 to 2200 m a.s.l. on the northern and southern slopes of the Guadarrama Mountains Fig, S1). In spring of 2019 (May), we sampled another 134 points at similar locations and elevation ranges (mean elevation, winter: 1358 m, spring: 1379 m; Student t test, t = – 0.42, p = 0.676). Passerines (O. Passeriformes) and similar species (e.g. woodpeckers and doves) were recorded either by hearing or sight at each sampling point during a period of 10 min within a 100-m-long radius (Johnson 2010). We excluded large species (e.g. raptors, crows, storks), because they are not properly recorded by these sampling points since their large home range may lead to the detection of a given individual at widely separated sampling points. We also excluded sparrows (Passer spp.) and starlings (Sturnus spp.) because they distribute around human settlements. Finally, to work with suitable sample sizes, only those species recorded in four or more sampling points in either season were included in further analyses. These results were used to calculate the mean abundance of each species (i) in spring (Asi) and winter (Awi) from sampling points (A = (Ʃ ai)/n, where ai is the species abundance in the i sampling point and n the sample size). After, these data were used to calculate the normalized difference of species abundances between spring and winter (a proxy of the species resistance to seasonal changes) by means of ΔAi (ΔAi = [Asi—Awi] / [Asi + Awi]).
Microhabitat use
We used the foraging substrate recorded by Carrascal et al. (1987) in the Guadarrama Mountains to assess microhabitat use (sensu Hall et al. 1997). These data refer to the percentage of records detected for focal individuals within a set of foraging substrata during winter (ground, shrubs, trunks, branches, cones, twigs, leaves and air). In addition, during the 2019 breeding period, we carried out additional fieldwork to obtain sufficient data for all the species considered in our study that were absent or very scarce during winter (Online Appendix 1). This sampling explicitly assumes that, despite the potential effect of predation risk, food availability or episodic hostile weather events which might affect the patterns, there are no sharp changes in the seasonal use of the microhabitat by the study species (Alatalo 1980). These data were used to perform a Principal Component Analysis with the species x substrata matrix to generate a latent variable (PCi) able to explain microhabitat use by bird species. The first component depicted a gradient from ground to canopy use (PC1, explained variance = 32.33%; eigenvalue = 2.57; factor loadings: – 0.600 ground, -0.005 cones, 0.154 shrubs, 0.223 trunks, 0.226 air, 0.407 leaves, 0.415 twigs and 0.422 branches) so that we could employ the factor scores of each species within this component as an index of increasing canopy use.
Taxonomic diversity
We used the number of subspecies within the Western Palaearctic (Boesman et al. 2019) without considering Macaronesia owing to the potential strong effect of island isolation on subspecies diversification (Phillimore et al. 2007). We followed the southern limit of the Palaearctic according to Roselaar (2006), which includes the northern Maghreb, eastern gulf of Sidra (Libya) and some northern parts of Syria, Iraq and Iran. To the east, the boundaries were set from the Urals (Russia) to northern Iran, eastern Caspian Sea coast including the whole Caucasus and Anatolia. It is important to note that this area is commonly considered an evolutionary scenario in which Mediterranean refuges have played a main role in population differentiation during the Pleistocene (Hewitt 2004, 2011). Finally, since the number of subspecies can be positively correlated to species range, we assessed the extent of the breeding range of species as a covariate in all analyses (Belliure et al. 2000; Phillimore et al. 2007; Martin and Tewksbury 2008; Kisel and Barraclough 2010). We used the available maps (e.g. Birdlife, http://www.birdlife.org) to recalculate the size of breeding ranges within the limits of the western Palearctic. Each species range was approximated in square kilometers by the sum of the area of the countries or regions covered by each species distribution and then log-transformed.
In the era of genomics, it is possible to use more powerful molecular methods to illustrate the diversification of species. However, this approach would be problematic for the current work, since not all the birds considered have been studied in a comparable way, but rather using a range of methods from classic genetic tools (e.g. mtDNA) to the state-of-the-art next-generation DNA sequencing (e.g. ddRADseq and whole-genome sequencing) (Parau and Wink 2021). Thus, any attempt to assess differentiation within species with just one methodology will face its own limitations when applied to a large set of species. In this context, it is important to point out that the phenotypic and genetic patterns do not have to be similar (Winker 2021), which leads us to less mechanical and more interpretive approaches to the diversity of populations and species (Sangster 2018). In this context, the number of subspecies accepted by influential checklists (e.g. Boesman et al. 2019) may reflect a consensual update of the observed within-species differentiation with which to identify evolutionary dynamics of phenotypic change (Zamudio et al. 2016).
Analyses
According to our conceptual framework, we tested (1) whether seasonal changes in abundance were related to microhabitat use (canopy dwellers will experience smaller changes than ground dwellers), (2) if the number of subspecies was related to microhabitat use after controlling for the effect of breeding range and (3) if the number of subspecies was related to a higher degree of seasonal variation in abundance after controlling for the effect of breeding range. To approach this, we used phylogenetic generalized least squares (PGLS) models to explore the relationship between taxonomic diversity and the explanatory variables while accounting for the effect of the phylogenetic relatedness of the study species. We used the caper 1.0.1 package in R (Orme 2018) on a consensus phylogenetic tree (computed by the method "least squares" in phytools 0.7–20; Revell 2012) resulting from 1000 draws (“Erickson All Species” backbone phylogeny, Ericson et al. 2006) supplied by the “Global Phylogeny of Birds” website (www.birdtree.org; Jetz et al. 2012). PGLS models resulting from tests 2 and 3 above were compared using the Akaike Information Criterion (AICc) for small samples (Burnham and Anderson 2004). PGLS analyses can account for the phylogenetic signal on the model residuals by means of different parameters, i.e. λ, δ and κ (see Symonds and Blomberg, 2014). In this case, we fixed δ and κ values to 1, whereas Pagel’s λ was calculated through maximum likelihood estimation.
Results
Microhabitat use of the 32 species considered in this study was assessed by factor scores, with positive and negative scores related to the use of the canopy and the ground, respectively (Table S1). The distribution of these scores was significantly related to changes in bird numbers resulting from seasonal dispersion (model 1, Table 1), supporting the prediction that canopy dwellers experienced weaker numerical changes during the summer–winter transition (Table 1). In addition, low seasonal changes in abundance were positively related to the number of subspecies after controlling for the effect of breeding range (model 2, Table 1). This suggests that canopy dwellers experienced higher rates of population differentiation. Finally, microhabitat use was able to predict the number of subspecies supporting the main effect of this trait on the population differentiation of forest birds (Table 1). Therefore, the results in this study support the hypothesized functional link between microhabitat use, resistance to seasonal changes in abundance and population differentiation in southwestern Palaearctic forest birds. Regarding Pagel’s λ, a phylogenetic signal was not found in any of the three models (Table 1). Hence, we can assume sample independence within the species set and the soundness of our analyses. To summarize the observed patterns, we arbitrarily classified the species as either canopy or ground exploiters (Fig. 1) by the positive or negative scores obtained along the gradient of microhabitat use (see Methods and Table S1 1). Both groups showed significant differences, with higher abundance changes in ground dwellers (F1,30 = 10.73, P = 0.003, λ = 0) and more subspecies in canopy dwellers (F1,30 = 5.12, P = 0.031, λ = 0).
Box-and-whisker diagrams of normalized differences in seasonal changes in abundance (1) and number of subspecies corrected by range size (2) between canopy- and ground-dweller birds. These birds have been arbitrarily classified as either canopy or ground exploiters by the positive or negative scores obtained along the gradient of microhabitat use defined by a principal component analysis (see Methods and Online Appendix 1). In both cases, the differences are statistically significant after accounting for the effect of the phylogenetic relatedness of the species (see text)
Discussion
The hypothesis that climatic stability selects for reduced dispersal and increases allopatric speciation may explain why highly seasonal temperate areas have fewer species than inter-tropical regions (Martin and Tewksbury 2008; Jocque et al. 2010; Belmaker et al. 2011; Salisbury et al. 2012). However, large-scale processes related to environmental stability may be buffered by other processes acting at smaller scales. In this context, it has been suggested that forest cover buffers the effect of climate in some micro-refugia allowing some species to persist in increasingly adverse conditions (Suggitt et al. 2018). Our results link both approaches to suggest that the use of some stable microhabitats by birds increases their ability to cope with seasonal changes, reduces dispersion in search of suitable wintering areas and increases species diversification in birds within temperate forests.
Winter harshness constrains bird populations that reside year-round in the northern hemisphere, as their winter survival depends primarily on obtaining enough food for self-maintenance in a context of tough climate conditions (Newton 1998, Doherty and Grubb 2002). This means that birds are more compelled to track food in winter, forcing them to move at different spatial scales, including altitudinal gradients (Pageau et al. 2020). This has been reported in the study area where some species breeding in mountain areas move to more southern areas in search of fruit-bearing shrubs (e.g. Sylvia atricapilla, Tellería and Pérez-Tris 2001), while others move locally in the search of forest food resources (e.g. tree-gleaning birds, Carrascal et al. 2012). The potential cause–effect link between microhabitat use and seasonal changes in abundance is backed by a set of adaptations that allows canopy dwellers to remain in breeding grounds during winter. In this case, most canopy dwellers are specialized in the search of invertebrates (spiders, insects) in leaves, lichens, or bark crevices (Ulfstrand 1977). In this way, while active invertebrates are a highly seasonal resource in temperate areas that impel many insectivorous birds to leave forests in winter (Belmaker et al. 2011), dormant invertebrates are a ubiquitous and stable food resource (Pettersson et al. 1995; Basile et al. 2020).
Since trophic niche has been reported to be very conservative in European birds (Pearman et al. 2013), it can be conjectured that habitat use has driven bird evolution during the last one million years. Most bird species occurring at this time in the southwestern Palaearctic (e.g. Johansson et al. 2018) were probably distributed into different refugia according to the advance and retreat of glaciers (Hewitt 2004, 2011). In this context, restrictions to gene flow among canopy dwellers between these refuges and the subsequent effect of divides among populations spreading northwards could have produced some of the current trends of subspecific differentiation in forest bird species.
Our results differ at first glance from the patterns detected in Neotropical birds, where the canopy dwellers are less diverse than ground dwellers (Burney and Brumfield 2009; Salisbury et al. 2012; Smith et al. 2014). This pattern has been explained by the fact that most canopy dwellers are vagrant birds that track fluctuating fruit resources while understory birds, most of them insectivores, are linked to more stable conditions (Salisbury et al. 2012; Smith et al. 2014; Miller et al. 2021). But, interestingly, the observed link between habitat use and population differentiation in temperate forests can also be explained in terms of habitat (microhabitat) stability. Thus, despite the differences, results in Palaearctic and Neotropical forest birds are similar, because they support the role of stable food related to some microhabitats as drivers of poor dispersal and population differentiation.
References
Alatalo RV (1980) Seasonal dynamics of resource partitioning among foliage-gleaning passerines in northern Finland. Oecologia 45:190–196. https://doi.org/10.1007/BF00346459
Basile M, Asbeck T, Jonker M, Knuff AK, Bauhus J, Braunisch V, Mikusisnki G, Storch I (2020) What do tree-related microhabitats tell us about the abundance of forest-dwelling bats, birds, and insects? J Environ Manage 264:110401. https://doi.org/10.1016/j.jenvman.2020.110401
Belliure J, Sorci G, Møller AP, Clobert J (2000) Dispersal distances predict subspecies richness in birds. J Evolution Biol 13:480–487
Belmaker J, Sekercioglu CH, Jetz W (2011) Global patterns of specialization and coexistence in bird assemblages. J Biogeogr 39:193–203. https://doi.org/10.1111/j.1365-2699.2011.02591.x
Boesman P, Bonan A, Christie D, Collar N, Copete JL, Elliott A, García E, de Juana E, Jutglar F, Kirwan G, Marks J, Sartagal J, Sharpe CJ (2019) Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona
Burney CW, Brumfield RT (2009) Ecology predicts levels of genetic differentiation in Neotropical birds. Am Nat 174:358–368. https://doi.org/10.1086/603613
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Method Res 33:261–304. https://doi.org/10.1177/0049124104268644
Busse P (2001) European passerine migration system–what is known and what is lacking. Ring 23: 3–36. https://www.wbwp-fund.eu/ring/pdf/23-1-2/bus1.pdf
Carrascal LM, Potti J, Sánchez-Aguado FJ (1987) Spatio-temporal organization of the bird communities in two Mediterranean montane forests. Holarctic Ecol 10:185–192. https://doi.org/10.1111/j.1600-0587.1987.tb00757.x
Carrascal LM, Palomino D (2012) Variación geográfica de la riqueza de especies invernantes en la península Ibérica. Estacionalidad y determinismo ambiental. In: del Moral JC, Molina B, Bermejo A, Palomino D (Ed). Atlas de las aves en invierno en España 2007–2010. Madrid, Spain: Ministerio de Agricultura, Alimentación y Medio Ambiente-SEO/BirdLife
Chapman BB, Brönmark C, Nilsson JA, Hansson LA (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775. https://doi.org/10.1111/j.1600-0706.2011.20131.x
Claramunt S, Derryberry EP, Remsen JV, Brumfield RT (2011) High dispersal ability inhibits speciation in a continental radiation of passerine birds. P Roy Soc B 279:1567–1574. https://doi.org/10.1098/rspb.2011.1922
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland
Ericson PG, Anderson CL, Britton T, Elzanowski A, Johansson US, Kallersjo M, Ohlson JI, Parsons J, Zuccon D, Myr G (2006) Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol Lett 2:543–547. https://doi.org/10.1098/rsbl.2006.0523
European Environment Agency (2011) European forest types: categories and types for sustainable forest management reporting and policy. Publications Office of the European Union, Copenhagen
Hall LS, Krausman PR, Morrison ML (1997) The habitat concept and a plea for standard terminology. Wildlife Soc 25: 173–182. http://www.jstor.org/stable/3783301
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. T Roy Soc 359:183–195. https://doi.org/10.1098/rstb.2003.1388
Hewitt GM (2011) Quaternary phylogeography: the roots of hybrid zones. Genetica 139:617–638. https://doi.org/10.1007/s10709-011-9547-3
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 15:444–448. https://doi.org/10.1038/nature11631
Jocque M, Field R, Brendonck L, De Meester L (2010) Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Global Ecol Biogeogr 19:244–252. https://doi.org/10.1111/j.1466-8238.2009.00510.x
Johansson US, Nylinder S, Ohlson JI, Tietze DT (2018) Reconstruction of the late Miocene biogeographical history of tits and chickadees (Aves: Passeriformes: Paridae): A comparison between discrete area analyses and probabilistic diffusion approach. J Biogeogr 45:14–25. https://doi.org/10.1111/jbi.13095
Johnson DH (2010) In Defense of Indices: The Case of Bird Surveys. J Wildlife Manage 72:857–868. https://doi.org/10.2193/2007-294
Kisel Y, Barraclough TG (2010) Speciation has a spatial scale that depends on levels of gene flow. Am Nat 175:316–334. https://doi.org/10.1086/650369
Martin PR, Tewksbury JJ (2008) Latitudinal variation in subspecific diversification of birds. Evolution 62:2775–2788. https://doi.org/10.1111/j.1558-5646.2008.00489.x
Miller MJ, Bermingham E, Turner BL, Touchon JC, Johnson AB, Winker K (2021) Demographic consequences of foraging ecology explain genetic diversification in Neotropical bird species. Ecol Lett 24:563–571. https://doi.org/10.1111/ele.13674
Orme D (2018) The caper package: comparative analysis of phylogenetics and evolution in R. Available at http://cran.rproject. org/web/packages/caper/vignettes/caper.pdf. (accessed 3rd April 2020)
Pageau C, Vale MM, de Menezes MA, Barçante L, Shaikh MS, Alves MA, Reudink MW (2020) Evolution of altitudinal migration in passerines is linked to diet. Ecol Evol 10:3338–3345. https://doi.org/10.1002/ece3.6126
Pârâu LG, Wink M (2021) Common patterns in the molecular phylogeography of western palearctic birds: a comprehensive review. J Ornithol. https://doi.org/10.1007/s10336-021-01893-x
Pearman PB, Lavergne S, Roquet C, Wüest R, Zimmermann NE, Thuiller W (2013) Phylogenetic patterns of climatic, habitat and trophic niches in a European avian assemblage. Global Ecol Biogeogr 23:414–424. https://doi.org/10.1111/geb.12127
Pettersson RB, Ball JP, Renhorn KE, Esseen PA, Sjöberg K (1995) Invertebrate communities in boreal forest canopies as influenced by forestry and lichens with implications for passerine birds. Biol Conserv 74:57–63. https://doi.org/10.1016/0006-3207(95)00015-V
Phillimore AB, Freckleton RP, Orme CDL, Owens IP (2006) Ecology predicts large-scale patterns of phylogenetic diversification in birds. Am Nat 168:220–229. https://doi.org/10.1086/505763
Phillimore AB, Orme CDL, Davies RG, Hadfield JD, Reed WJ, Gaston KJ, Freckleton RP, Owens IP (2007) Biogeographical basis of recent phenotypic divergence among birds: a global study of subspecies richness. Evolution 61:942–957. https://doi.org/10.1111/j.1558-5646.2007.00068.x
Revell LJ (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol 38:231–253. https://doi.org/10.1146/annurev.ecolsys.38.091206.095611
Roselaar KCS (2006) The boundaries of the Palearctic region. Brit Birds 99:602–618
Salisbury CL, Seddon N, Cooney CR, Tobias JA (2012) The latitudinal gradient in dispersal constraints: ecological specialisation drives diversification in tropical birds. Ecol Lett 15:847–855. https://doi.org/10.1111/j.1461-0248.2012.01806.x
Sangster G (2018) Integrative taxonomy of birds: The nature and delimitation of species. In: Tietze DT (Ed.) Bird Species, Springer, Cham, pp 9–37 https://doi.org/10.1007/978-3-319-91689-7_2
Schweizer M, Liu Y (2018) Avian diversity and distributions and their evolution through space and time. In: Tietze DT (Ed.) Bird Species, Springer, Cham, pp 129–145. https://doi.org/10.1007/978-3-319-91689-7_8
Slatkin M (1987) Gene flow and the geographic structure on natural populations. Science 236:787–792. https://doi.org/10.1126/science.3576198
Smith BT, McCormack JE, Cuervo AM, Hickerson MJ, Aleixo A, Cadena CD, Pérez-Emán J, Burney CW, Xie X, Harvey MG, Faircloth BC, Glenn TC, Derryberry EP, Prejean J, Fields S, Brumfield RT (2014) The drivers of tropical speciation. Nature 515:406–409. https://doi.org/10.1038/nature13687
Suggitt AJ, Wilson RJ, Isaac NJB, Beale CM, Auffret AG, August T, Bennie JJ, Crick HQP, Duffield S, Fox R, Hopkins JJ, Macgregor NA, Morecroft MD, Walker KJ, Maclean IM (2018) Extinction risk from climate change is reduced by microclimatic buffering. Nat Clim Change 8:713–717. https://doi.org/10.1038/s41558-018-0231-9
Symonds E, Blomberg SP (2014) A Primer on Phylogenetic Generalised Least Squares. In: Garamszegi L (ed) Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. Springer, Cham, pp 105–130
Tellería JL, Pérez-Tris J, Carbonell R (2001) Seasonal changes in abundance and flight-related morphology reveal different migration patterns in Iberian forest passerines. Ardeola 48:27–46
Ulfstrand S (1977) Foraging niche dynamics and overlap in a guild of passerine birds in a south Swedish coniferous woodland. Oecologia 27:23–45. https://doi.org/10.1007/BF00345683
Winker K (2021) An overview of speciation and species limits in birds. Ornithology. https://doi.org/10.1093/ornithology/ukab006
Zamudio KR, Bell RC, Mason NA (2016) Phenotypes in phylogeography: Species’ traits, environmental variation, and vertebrate diversification. Proc Natl Acad Sci 113:8041–8048. https://doi.org/10.1073/pnas.1602237113
Acknowledgements
The study was supported by the project CGL2017-85637-P of the Spanish Ministry of Science and Innovation. Four anonymous reviewers considerably improved an early version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Aleixo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talavera, A., Tellería, J.L. Does microhabitat use affect population differentiation? A test with southwestern Palaearctic forest birds. J Ornithol 163, 923–929 (2022). https://doi.org/10.1007/s10336-022-01998-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-01998-x