Abstract
The brood parasitic Common Cuckoo Cuculus canorus is best known for its two-note “cu-coo” call which is almost continuously uttered by male during the breeding season and can be heard across long distances in the field. Although the informative value of the cuckoo call was intensively investigated recently, it is still not clear whether call characteristic(s) indicate any of the phenotypic traits of the respective vocalising individuals. To fill this gap, we studied whether the call rate of male cuckoos (i.e., the number of calls uttered per unit of time) provides information on their body size, which might be a relevant trait during intrasexual territorial conflicts. We captured free-living male cuckoos and measured their body size parameters (mass, wing, tail and tarsus lengths). Each subject was then radio-tagged, released, and its individual “cu-coo” calls were recorded soon after that in the field. The results showed that none of the body size parameters covaried statistically with the call rates of individual male Common Cuckoos. In addition, we experimentally tested whether the “cu-coo” call rates affect behavioural responses of cuckoos using playbacks of either a quicker or a slower paced call than the calls with natural rates. Cuckoos responded similarly to both types of experimental playback treatments by approaching the speaker with statistically similar levels of responses as when presented with calls at the natural rate. We conclude that male Common Cuckoos do not advertise reliable information acoustically regarding their body size, and so, cuckoo calls are neither useful to characterize cuckoos’ phenotypic traits directly nor to indicate environmental quality indirectly.
Zusammenfassung
Von der Rufrate des Kuckucks lässt sich weder auf die Körpergröße noch auf die Reaktionen von Artgenossen auf das Vorspielen der Rufe schließen
Der Brutparasit Kuckuck (Cuculus canorus) ist vor allem für seinen Ruf „gu-kuh“ bekannt, der während der Brutzeit von den Männchen fast ununterbrochen ausgestoßen wird und im Freiland über weite Entfernungen zu hören ist. Obwohl der Informationsgehalt des Kuckuckrufs in jüngster Zeit intensiv untersucht wurde, ist immer noch nicht klar, ob die Rufcharakteristik(en) auf irgendwelche phänotypischen Merkmale des rufenden Individuums hinweisen. Um diese Lücke zu schließen, untersuchten wir, ob die Rufrate männlicher Kuckucke (d.h. die Anzahl der pro Zeiteinheit geäußerten Rufe) Informationen über ihre Körpergröße liefert, was innerhalb des gleichen Geschlechts ein für Revierkonflikte relevantes Merkmal sein könnte. Wir fingen freilebende männliche Kuckucke und maßen ihre Körpergrößenparameter (Masse, Flügel-, Schwanz- und Tarsuslänge). Jedes Tier wurde dann mit einem Sender versehen, wieder freigelassen und seine individuellen Rufe kurz darauf im Freiland aufgezeichnet. Die Ergebnisse zeigten, dass keiner der Körpergrößenparameter statistisch mit den Rufraten einzelner männlicher Kuckucke kovariierte. Zusätzlich testeten wir experimentell, ob die „gu-kuh“-Rufraten die Reaktionen anderer Kuckucke beeinflussten, indem wir die Wiedergabe der Rufe entweder schneller oder langsamer als mit den natürlichen Rufraten abspielten. Die Kuckucke reagierten im Experiment auf beide Wiedergabegeschwindigkeiten ähnlich, indem sie sich dem rufenden Vogel mit statistisch ähnlichen Rufraten näherten. Wir schließen daraus, dass männliche Kuckucke auf akustischem Weg keine verlässlichen Informationen über ihre Körpergröße abgeben und ihre Rufe daher nicht geeignet sind, direkt auf phänotypische Merkmale schließen zu lassen oder indirekt Informationen über die Umweltqualität zu geben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic communication is fine-tuned across diverse avian lineages. Auditory signals, including songs and calls, can carry information both about and for conspecifics and/or heterospecifics (Bradbury and Vehrencamp 2011). Typically, learned songs offer complex ways to express vocal signals, but are restricted to a handful, species-rich lineages, including songbirds, hummingbirds, and parrots. Many other bird taxa communicate with simpler and non-learned vocalisations, termed calls (Brenowitz 1991; Kumar 2003). The calls and songs of individual birds often show variation within a population (Catchpole and Slater 2008), depend on several factors (e.g., age, sex, body size, and nutritional condition), and may function as honest signals of the individual quality of the signaller (Galeotti et al. 1997; Redpath et al. 2000; Murphy et al. 2008). For example, body size of birds may reliably affect songs’ or calls’ acoustic characteristics (Hall et al. 2013; Derryberry et al. 2018; but also see Brumm 2009; Patel et al. 2010; Budka and Osiejuk 2013).
Common Cuckoos Cuculus canorus (hereafter: cuckoos) are obligate brood parasites, utilising other avian species for incubating, feeding, and rearing their offspring (Makatsch 1955; Payne 2005; Erritzoe et al. 2012). Cuckoos exhibit complex social lives (Davies 2000), probably as the consequence of being emancipated from several costly and time-consuming forms of parental care. The vocalisation of cuckoos is known to play an important part in conspecific communication, for example, in territorial disputes both between males (Moskát et al. 2017; Yoo et al. 2020), but also between females (Deng et al. 2019a; Moskát and Hauber 2019; Xia et al. 2019).
Male cuckoos utter almost continuously their characteristic “cu-coo” calls during the breeding season (Wyllie 1981); therefore, they can be detected relatively easily. Previous studies attempted to use the cuckoos’ presence in an area as an indicator of the naturalness (i.e., the opposite of anthropogenic disturbance) of the habitat, including as a predictor avian species richness (Morelli et al. 2015) and diversity (Tryjanowski and Morelli 2015). Although several factors may affect cuckoo calling behaviour (Benedetti et al. 2018), this line of studies treated the length of continuous calling as a positive predictor of avian species richness (Benedetti et al. 2018) or individual cuckoo quality (Møller et al. 2016). As vocal production is metabolically costly (e.g., Oberweger and Goller 2001), individuals may advertise their higher quality by decreasing the amount of time elapsed between two calls (i.e., increasing call rate), or increasing the speed of the uttered calls, both of which result in higher number of calls per unit of time. Call rate is an honest indicator of body size and condition in several bird species (e.g., Redpath et al. 2000; Garamszegi et al. 2004); therefore, it is also expected to be a suitable signal to advertise individual quality (i.e., phenotype) in cuckoos.
Although body size or condition may affect acoustic parameters of calling also in cuckoos, no study analysed this potential relationship previously in this particular species. Larger body size might have advantages for cuckoos, as larger-bodied birds tend to have larger territories and are capable of producing louder acoustic signals (Catchpole and Slater 2008). Here we compared body size parameters (mass, and tarsus, wing, and tail sizes) and an estimated body condition index with the call rate in free-living male Common Cuckoos, tagged with telemetry transmitters. We hypothesised that larger individuals (e.g., heavier in weight or with greater body length parameters) produced calls more dynamically, i.e., with higher call rates than others that are smaller. Previous studies suggested indirectly that body condition may affect the syllabus production rate of cuckoos (e.g., Tryjanowski et al. 2018), and therefore, to test if the call rates have a signalling value between male cuckoos, we carried out a field experiment, by playing back calls at natural or modified (i.e., quicker and slower) rates. Here we predicted that territorial males would respond more aggressively to the playbacks with higher call rates (i.e., quicker) than to those of lower call rates (i.e., slower variant).
Materials and methods
Study area
Field work was conducted in a 20 × 30 km area around Apaj, central Hungary (47° 6′ 53.9″ N; 19° 5′ 21.2″ E), ca. 50 km south of Budapest, in May between 2016 and 2019. Here Common Cuckoos parasitize Great Reed Warblers Acrocephalus arundinaceus in the reedbeds of a network of small irrigation and flood relief canals, connected with the river Danube. This host species prefers reedbed edges and other edge-like habitats along the canals for breeding (Moskát and Honza 2002; Mérő et al. 2015). Common Cuckoos typically perch on and move between the trees along the banks of the canals, holding partly overlapping territories (Moskát et al. 2019).
Field procedures
We captured Common Cuckoos with mist-nets using playbacks of male and female cuckoos to attract them. Upon capture, we sexed cuckoos by morphological characters (Svensson et al. 2010; also confirmed by DNA analysis in 2016 and 2017; Moskát et al. 2019), then we measured the following body size metrics: body mass (g; to the nearest 1 g with a Pesola spring scale), wing and tail length (mm; to the nearest 1 mm, with a ruler), and tarsus length (to the nearest 0.1 mm; with a calliper). Following measurements, we tagged each cuckoo with a 1.2 g (1% of the body mass) radio transmitter (Pip3, type 392 by Biotrack Ltd; for more details, see Moskát et al. 2017) and released them at the site of capture.
In the field we followed the tagged cuckoos and identified them individually using a Sika receiver (Biotrack Ltd.) and a flexible 3-element Yagi antenna (Biotrack Ltd.). Calls of male cuckoos were recorded 2–5 days after they had been captured (mean ± SD = 3.21 ± 2.05) and tagged, using a Telinga Universal parabola dish with Rycota Hi Wind Cover, a Marantz PMD-620 MKII sound recorder (48 kHz sampling rate, 24-bit quality, wav format), a Sennhesiser ME62 microphone, with a K6 powering module and a FEL MX mono preamp.
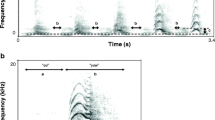
We calculated the natural call rate of cuckoos by measuring the number of continuously uttered calls divided by the length in sec. To obtain a representative sample, we chose sound recordings randomly (n = 18), where the calling sequence was complete, used for assessing our call rate measurements on radio-tagged cuckoos (n = 23). As call rate could be measured in several ways, e.g., by dividing the number of calls per time for a full recording, or a section of continuous calling including short pauses and/or other call types than “cu-coo” (e.g., the 3-note “cu-cu-coo”, c.f. Xia et al. 2019), we chose complete sequences with no pause and containing only the “cu-coo” call type (Fig. 1). We also took care not to sample just after a female conspecific’s bubbling call (Chance 1940; Wyllie 1981) or avoiding any other potential effect might impact the tempo of calling (e.g., the arrival of a new male or female cuckoo).
Wyllie (1981) mentioned that this species’ males uttered 10–20 (up to 270) calls in uninterrupted series, with short inter-series intervals. Møller et al. (2017) measured a range of 1–45 repeats (mean ± SE: 15.6 ± 2.0) in 24 male cuckoos. We did not analyse the maximum number of syllables uttered continuously, which had been suggested to be an indicator of individual quality in male Common Cuckoos (Møller et al. 2016; Tryjanowski et al. 2018). We did not opt for this approach, because cuckoos live at high densities in our study area (Moskát and Honza 2002), and many conspecifically initiated social interactions disrupt continuous callings of individual male cuckoos at our study site (CM pers. obs.). Nonetheless, we observed the longest calling sequences from solitary, newly arriving male cuckoos (over 100 calls per series, where similar series were repeated several times after a pause of few seconds). Consequently, the numbers of syllables uttered continuously are unlikely to function as honest indicators of body size or condition (sensu Maynard Smith and Harper 2003) in our study area with high cuckoo density. Instead, here we used the temporal frequency of the calls uttered during unit of time (i.e., call rate) as a proxy for this metric (e.g., Yorzinski and Vehrencamp 2009; Carlson et al. 2017). We also conducted a playback experiment manipulating this trait to elicit behavioural responses of territorial male cuckoos (see below).
Playback experiment
We carried out a field experiment to test the function of one specific acoustic variable (call rate, i.e., the number of calls uttered per unit of time (s)) expected to be associated with body size and/or condition (Podos 1997; Martin et al. 2011; Weiss et al. 2012; Nishida and Takagi 2018), in a territorial context. We manipulated original cuckoo call recordings either by reducing or increasing the length of pauses among syllables, producing “quicker” or “slower” audio files for playbacks (Fig. 1), and we also used behavioural response data to cuckoo call sequences played back at the natural, unmanipulated speed (“normal”) from 2016. As the “cu-coo” call’s main function is territorial defence (Moskát et al. 2017; Tryjanowski et al. 2018), we also expected that the rate of cuckoo calls affected territorial display efficiency. Consequently, the playback file with higher or lower call rates may attract more or fewer conspecific males, respectively.
Here, we utilised the file structure we already used in previous experiments (2-min audio files, containing 3 × 30 s sequence of syllables, and 15 s pauses among the sequences; see more details of the basic call playback file structure in Moskát et al. 2017). The playback files with “normal” speed contained 20.4 ± 0.76 (mean ± SE; range: 9–24) calls in the basic, 30 s, unit of the playback files, the “quicker” files had 28.0 ± 1.3 calls (range: 18–36), and the “slower” files contained 15.2 ± 0.69 calls (range: 9–18). The number of calls per unit of time differed both in the “quicker” and “slower” files when compared to the calling frequency in the “normal” file (Mann–Whitney U test: quicker vs. normal: z17,15 = − 3.888, P < 0.001; slower vs. normal: z16,15 = − 3.959, P < 0.001). Playback files were constructed with the Audacity 2.1.0. program, and we manipulated call rate by reducing or increasing inter-call intervals. The “quicker” vs. “slower” comparison of call numbers was, of course, also highly significant (z17,16 = − 4.843, P < 0.001).
For the call rate experiment we searched for playback sites along the wooded parts of irrigation canals inhabited by host Great Reed Warblers in a slow-moving car. Experimental trials were initiated at sites, where a male cuckoo was heard and seen within 80 m. For playback, we used a JBL Xtreme (40 W) loudspeaker, connected to a Lenovo TAB 2 A7 tablet with a 20 m audio cable (see more technical details of the playback in Moskát et al. 2017). The loudspeaker was placed on a tree ~ 1.5 m height, and two observers handled the equipment and observed wild cuckoos while hiding behind a bush. Observations on cuckoos were dictated onto a Tascam dr-05 ver2 sound recorder. To avoid pseudoreplication (sensu Hurlbert 1984; Kroodsma 1989) we played a sound file only once, and selected the consecutive trial sites for playback at least 1 km distance from each other along the canals to use an individual focal cuckoo only once (e.g., Moskát et al. 2017). Playback experiments with quicker and slower treatments were carried out between May 6 and 11, 2018, in the early hours of the day (between 6 and 11 h). We also used data from playbacks of normal-speed cuckoo calls as a control for the speed manipulation specifically, and a harmless, similarly sized sympatric species, the Eurasian Collared Dove (Streptopelia decaocto) from May 2016 (Moskát et al. 2017) as a positive control, following a similar protocol to the experimental trials described above. All playbacks were conducted under good weather conditions, avoiding rainy, windy, or hot periods of the days. The sex of cuckoos was identified by their sex-specific calls and the partially sexually dichromatic plumage characteristics in this species (e.g., Moskát et al. 2020).
We analysed the following behavioural variables to characterize the cuckoos’ responses during the experimental playback trials in two sets of tests:
(i) A robust comparison of the effects of call rate for the categories “quicker”, “normal”, and “slower”, together with the dove calls used for general control, as these have proven to be the most important responses to playbacks in previous experiments on the Common Cuckoo (e.g., Moskát et al. 2017; Moskát and Hauber 2019).
Movements: A binary variable expressing if the focal cuckoo approached the speaker during the 2-min playback (Y/N).
Closest distance (m): The closest value of distance when the focal bird approached the speaker during a playback trial, either by flying or sitting on a nearby tree.
(ii) A more detailed comparison of call rate modulation (for the categories “quicker” and “slower”) to reveal fine-tuned differences in Common Cuckoos’ responses:
Distance to first detection (m): The distance of a cuckoo from the speaker when it appeared or called in the vicinity of the speaker.
Latency of first detection (s): Time spent from the start of playback until the first visual or vocal appearance of the cuckoo in the vicinity of the speaker.
Closest distance (m): The shortest distance between the cuckoo and the speaker observed during the full playback period. Approaching the speaker closely can be regarded the most important variable indicating positive response to cuckoo playbacks (Moskát et al. 2017).
Latency of closest detection (s): Time spent from the start of playback until the closest appearance of the cuckoo to the speaker during the observational period.
Latency of calling (s): Time spent from the start of playback until the male cuckoo started calling.
Length of continuous calling (s): The longest continuous calling sequence within the observational period.
Number of flights: Number of flights of the focal bird during the observational period. Cuckoos often flew away or towards the speaker, above it, or flew circle-like routes around the speaker, then sat on a tree. Some of them later repeated the same movement(s) once or several times.
Number of birds: The number of male cuckoos observed in the vicinity of the speaker during the playback.
Statistical analyses
In addition to using body size metrics, to characterise body condition, we used the residual index (Gould 1975), where the body mass is regressed on body size, and the residuals provide an estimate of condition (e.g., Jakob et al. 1996). Using this approach, we offset the size effect per se in the estimation of the physiological condition state of each subject, thus this index reflects to the true body mass of an individual without size constraints; we further we refer as physiological condition index (PCI).
We compared the two sets of call rates with Mann–Whitney U test in SPSS ver. 17.0 (SPSS Inc., Chicago, IL). We also used the glm function in the R 3.6.1 package (R core team 2019) for generalised linear model with quasi-Poisson error term (glm, Bolker et al. 2009), where call rate was the dependent variable, year was the covariate, and body size parameters (mass, PCI, tarsus, wing, and tail lengths) as fixed effects. Year and the linear predictor were adjusted by the number of birds/year. Data on body sizes collected in 2016 (n = 6 from the total 29) were omitted from glm analyses, due to the lack of tarsus data from that year. We also included second order interaction terms in the model, i.e., years × body size parameters.
We also used binary logistic regression to compare cuckoos’ behaviour to the playbacks with call rate manipulation. In the model we used the playback type (“quicker” / “slower”) as dependent variable, and eight variables as independent variables (see list of variables in Table 2). The SPSS Statistics 17.0 program package was used for binary logistic analysis, selecting the method enter. This package was also used for calculating other statistical properties and parametric unpaired t test and non-parametric Kruskall–Wallis test. Principal component analysis (PCA) was used to analyse behavioural response variables of cuckoos to playback. PCA was started from the correlation matrix, and a component was retained if the corresponding eigenvalue was greater than 1.0. For ordination plots the first two components were used with no rotation on component loadings.
Fisher’s exact tests were carried out by the “vassarstats” online calculator (https://vassarstats.net/index.html; accessed on December 22, 2020).
Results
Call rate, body size and condition
We measured the call rate in vocalisation series uttered by radio-tagged cuckoos (call rate (no. of calls/sec): mean ± SD: 0.772 ± 0.084, n = 23). These values did not differ from values in a control data set on non-experimental and not radio-tagged birds (call rate (no. of calls/sec): mean ± SD: 0.786 ± 0.109, n = 18; Mann–Whitney U test: z = − 0.775, P = 0.438). Then, we tested if body size and condition metrics of cuckoos were associated with the call rate. Our generalised linear model revealed no such effects. Although cuckoo body mass, but not the other body size measures (wing, tail and tarsus length), showed a significant correlation with call rate in the full model, this effect was not stable and disappeared from the reduced models (Table 1, Table SM1, Supplementary Material).
Playback experiment
For the call rate experiment, we conducted 62 playbacks, including 17 trials with the quicker cuckoo call playbacks, 15 with normal rate, 16 of the slower call playbacks, and 14 Collared Dove controls. Male cuckoos responded to the playbacks of each of the conspecific male playback type in 94% and 100% of trials in the “slower” and “quicker” categories, respectively, and also in 100% in the “normal” category (Fisher’s exact test: P = 0.646), but only in 14% of trials to the dove controls (all cuckoos vs. dove controls, P < 0.001; Fig. 2a). We detected a similar pattern for the “closest distance” response variable (Fig. 2b): cuckoos approached the speaker closely in both the quicker and slower playback types, and when also calling sequence with the “normal” call rate was played (Kruskal–Wallis test: χ17,15,162 = 2.415, P = 0.299). Cuckoo responses to all cuckoo playback files (“quicker”, “normal” and “slower”) combined for closest distance significantly differed from cuckoo responses to control playbacks with dove calls (Mann–Whitney U test: z48,14 = − 4.940, P < 0.001).
a Percentage of playback experimental trials, where Common Cuckoos approached the speaker in response to four types of playbacks: “quicker”, “normal” or “slower” male Common Cuckoo male cu-coo calls and calls of Eurasian collared doves (“control”). Sample sizes are shown above the bars. b Closest distances of male Common Cuckoos approaching the speakers during the four types of playbacks (as above). Significant differences, tested by unpaired t test, are shown by asterisk (P < 0.001), or NS, when the test was not significant (P > 0.05). Standard errors of the means (SE) are shown by the whiskers
We also compared the two focal call rate modulation treatments, the “quicker” and “slower” playbacks, in detail. We did not detect any significant effect among the independent variables related to the “quicker” and “slower” trials in the binary logistic model as it showed that cuckoos responded to the two playback files similarly (Table 2). A PCA on the response variables revealed that the first two components accounted for the 58% of the total variance (Table 3), and the response variables were separated well along the two axes (Fig. 3). However, the “quicker” and the “slower” groups did not separate from each other in the PCA space when component scores were considered (independent sample t test, PCI: t31 = 0.495, P = 0.624; PCII: t31 = − 0.462, P = 0.647).
Discussion
Our study did not reveal any statistical relationships between the calling rate of individually tagged male Common Cuckoos and their body size parameters (mass, tarsus, wing and tail lengths, or PCI). Although avian song rate and repertoire size can positively covary with and/or signal individual quality (e.g., Nishida and Takagi 2018; reviewed in Catchpole and Slater 2008), these relationships do not hold in generality (e.g., Patel et al. 2010; Cardoso 2011; Mason and Burns 2015). Vocalisations of the cuckoos, and of many other brood parasitic species belonging to Cuculiformes, are simple and thought to be non-learned, i.e., inherited (Brenowitz 1991; Jarvis et al. 2000). However, call rate may be a vocal motor performance related trait, allowing a narrow range of acoustic variation to be affected by body condition. Van Hout et al. (2012) reported that in the European Starling Sturnus vulgaris it was not body condition but nutritional state that predicted vocalisation traits. Our anecdotal observations may support this idea in that during rainy, windy, and cold weather cuckoos at our study site preferred to stay in nearby forest, used for feeding or resting areas (Moskát et al. 2019), where they typically remained silent under these poor weather conditions (pers. obs.). Therefore, acute nutritional status may likely not have affected the direction and pattern of variations detected in our call measurements, as these calls had been recorded mostly under fair, sunny weather conditions. Future studies may reveal how diseases, for example bacterial, viral, or fungal infections, as well as any other health ailments, especially those affecting sound-producing organs (e.g., syringeal muscles), may modify cuckoos’ acoustic displays. Ecto-parasite load could be one of the most important factors, which is known to affect singing behaviour in diverse other bird species (e.g., Buchanan et al. 1999; Redpath et al. 2000), and can drive both visual and acoustic signalling (Garamszegi et al. 2004).
Our experiment with manipulated call rates also revealed no difference in how territorial male cuckoos responded to quicker, normal, or slower sequences of calls. This reflects a high inherent intrasexual signalling value of the male cuckoos’ characteristic cu-coo calls. It also suggests that, if any at all, signals other than acoustic displays may communicate body conditions better. The male cuckoos’ swift flights within their territories, physical fighting abilities, and visual displays of their prominent tail spots are among the potential signalling cues which could express cuckoos’ body condition and advertise it for nearby males as competitors and females as potential mating partners.
Previous studies revealed that Pale-winged Starlings Onychognathus nabouroup are able to modulate calling rate and use it for signalling on intending flight departure when foraging in pairs (Hausberger et al. 2020). Our results instead revealed that Common Cuckoos’ calling rates seem to be stable at a time. We also showed that call rate was not correlated with any of the body size parameters. In the playback experiment with quicker, normal, and slower tempo of “cu-coo” calls we revealed no statistical difference in responses to simulated territorial intrusions by playback type. Although these results are straightforward, we are aware that our sample sizes are relatively small, in terms of individual subjects. Previous studies (Zsebők et al. 2017) from the same area revealed that the potential sample size for individual parasitic subjects is constrained due to the limited number of available host nests, thus any research can be restricted, regardless of the fact that this area consistently had some of the highest rates of host-parasitism by Common Cuckoos across its distribution (Moskát and Honza 2002). As cuckoos' vocal activity may change daily and seasonally during their breeding season (Deng et al. 2019b; Yoo et al. 2020), we suggest that further studies should collect longer calling sequences at the different reproductive stages, accordingly. Apart from call rate, rhythm patterns may also have intraspecific signalling content, as was found in the Corncrake (Crex crex; Budka and Osiejuk 2013).
We conclude that male cuckoos do not acoustically advertise reliable information content of body size (probably linked to individual quality, condition and health state), and so the rate of cuckoo calls cannot be regarded as an honest signal of their body size and state or indicators of the cuckoo’s species-specific environmental quality.
References
Benedetti Y, Slezak K, Møller AP, Morelli F, Tryjanowski P (2018) Number of syllables in cuckoo Cuculus canorus calls: A test using a citizen science project. Sci Rep 8:12872. https://doi.org/10.1038/s41598-018-31329-1
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates Inc., Sunderland
Brenowitz EA (1991) Evolution of the vocal control system in the avian brain. Sem Neurosci 3:339–407. https://doi.org/10.1016/1044-5765(91)90030-R
Brumm H (2009) Song amplitude and body size in birds. Behav Ecol Sociobiol 63:1157–1165. https://doi.org/10.1007/s00265-009-0743-4
Buchanan KL, Catchpole CK, Lewis JW, Lodge A (1999) Song as an indicator of parasitism in the sedge warbler. Anim Behav 57:307–314. https://doi.org/10.1006/anbe.1998.0969
Budka M, Osiejuk TS (2013) Formant frequencies are acoustic cues to caller discrimination and are a weak indicator of the body size of Corncrake males. Ethology 119:960–969. https://doi.org/10.1111/eth.12141
Cardoso GC (2011) Paradoxical calls: the opposite signaling role of sound frequency across bird species. Behav Ecol 23:237–241. https://doi.org/10.1093/beheco/arr200
Carlson NV, Healy SD, Templeton CM (2017) A comparative study of how British tits encode predator threat in their mobbing calls. Anim Behav 125:77–92. https://doi.org/10.1016/j.anbehav.2017.01.011
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Chance EP (1940) The truth about the cuckoo. Country Life Ltd., London, UK
Davies NB (2000) Cuckoos, cowbirds and other cheats. Poyser, London
Deng Z, Lloyd H, Xia C, Møller AP, Liang W, Zhang Y (2019a) Components of variation in female common cuckoo calls. Behav Process 158:106–112. https://doi.org/10.1016/j.beproc.2018.10.007
Deng Z, Lloyd H, Xia C, Li D, Zhang Y (2019b) Within-season decline in call consistency of individual male Common Cuckoos (Cuculus canorus). J Ornithol 160:317–327. https://doi.org/10.1007/s10336-019-01631-4
Derryberry EP, Seddon N, Derryberry GA, Claramunt S, Seeholzer GF, Brumfield RB, Tobias JA (2018) Ecological drivers of song evolution in birds: disentangling the effects of habitat and morphology. Ecol Evol 8:1890–1905. https://doi.org/10.1002/ece3.3760
Erritzøe J, Mann CF, Brammer FP, Fuller RA (2012) Cuckoos of the world. Christopher Helm Publishers Ltd, London, UK
Galeotti P, Saino N, Sacchi R, Møller AP (1997) Song correlates with social context, testosterone and body condition in male barn swallows. Anim Behav 53:687–700. https://doi.org/10.1006/anbe.1996.0304
Garamszegi LZ, Møller AP, Török J, Michl G, Péczely P, Richarde M (2004) Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol 15:148–157. https://doi.org/10.1093/beheco/arg108
Gould S (1975) Allometry in primates, with emphasis on scaling and evolution of the brain. Contrib Primatol 5:244–292
Hall ML, Kingma SA, Peters A (2013) Male songbird indicates body size with low-pitched advertising songs. PLoS ONE 8:e56717. https://doi.org/10.1371/journal.pone.0056717
Hausberger M, Giacalone A, Harmand M, Craig AJFK, Henry L (2020) Calling rhythm as a predictor of the outcome of vocal interactions: flight departure in pale-winged starling pairs. Sci Nat 107:2. https://doi.org/10.1007/s00114-019-1658-1
Hurlbert SH (1984) Pseudoreplication and design of ecological field experiments. Ecol Monogr 54:187–211. https://doi.org/10.2307/1942661
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV (2000) Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature 406:628–632. https://doi.org/10.1038/35020570
Kroodsma DE (1989) Suggested experimental designs for song playbacks. Anim Behav 37:600–609. https://doi.org/10.1016/0003-3472(89)90039-0
Kumar A (2003) Acoustic communication in birds. Resonance 8:44–55. https://www.ias.ac.in/article/fulltext/reso/008/06/0044-0055
Makatsch W (1955) Der Brutparasitismus in der Vogelwelt. Neumann Verlag, Radebeul und Berlin
Martin JP, Doucet SM, Knox RC, Mennill DJ (2011) Body size correlates negatively with the frequency of distress calls and songs of Neotropical birds. J Field Ornithol 83(2):59–268. https://doi.org/10.1111/j.1557-9263.2011.00329.x
Mason NA, Burns KJ (2015) The effect of habitat and body size on the evolution of vocal displays in Thraupidae (tanagers), the largest family of songbirds. Biol J Linn Soc 114:538–551. https://doi.org/10.1111/bij.12455
Maynard Smith J, Harper D (2003) Animal signals. Oxford University Press, Oxford, UK
Mérő TO, Žuljević A, Varga K, Lengyel S (2015) Habitat use and nesting success of the Great Reed Warbler Acrocephalus arundinaceus in different reed habitats in Serbia. Wilson J Ornithol 127:477–485. https://doi.org/10.1676/13-200.1
Møller AP, Morelli F, Mousseau TA, Tryjanowski P (2016) The number of syllables in Chernobyl cuckoo calls reliably indicate habitat, soil and radiation levels. Ecol Ind 66:592–597. https://doi.org/10.1016/j.ecolind.2016.02.037
Møller AP, Morelli F, Tryjanowski P (2017) Cuckoo folklore and human well-being: cuckoo calls predict how long farmers live. Ecol Ind 72:766–768. https://doi.org/10.1016/j.ecolind.2016.09.006
Morelli F, Jiguet F, Reif J, Plexida S, Valli AS, Indykiewicz P, Simova P, Tichit M, Moretti M, Tryjanowski P (2015) Cuckoo and biodiversity: testing the correlation between species occurrence and bird species richness in Europe. Biol Conserv 190:123–132. https://doi.org/10.1016/j.biocon.2015.06.003
Moskát C, Hauber ME (2019) Sex-specific responses to simulated territorial intrusions in the common cuckoo: a dual function of female acoustic signaling. Behav Ecol Sociobiol 73:60. https://doi.org/10.1007/s00265-019-2665-0
Moskát C, Honza M (2002) European cuckoo Cuculus canorus parasitism and host’s rejection behaviour in a heavily parasitized Great Reed Warbler Acrocephalus arundinaceus population. Ibis 144:614–622. https://doi.org/10.1046/j.1474-919X.2002.00085.x
Moskát C, Elek Z, Bán M, Geltsch N, Hauber ME (2017) Can Common Cuckoos discriminate between neighbours and strangers by their calls? Anim Behav 126:253–260. https://doi.org/10.1016/j.anbehav.2017.02.013
Moskát C, Bán M, Fülöp A, Bereczki J, Hauber ME (2019) Bimodal habitat use in brood parasitic Common Cuckoos (Cuculus canorus) revealed by GPS telemetry. Auk 136:1–12. https://doi.org/10.1093/auk/uky019
Moskát C, Hauber ME, Růžičková J, Marton A, Bán M, Elek Z (2020) Female-female aggression and male responses to the two colour morphs of female Common Cuckoos. Sci Nat 107:28. https://doi.org/10.1007/s00114-020-01680-3
Murphy MT, Sexton K, Dolan AC, Redmond LJ (2008) Dawn song of the eastern kingbird: an honest signal of male quality? Anim Behav 75:1075–1084. https://doi.org/10.1016/j.anbehav.2007.08.020
Nishida T, Takagi M (2018) Song performance is a condition-dependent dynamic trait honestly indicating the quality of paternal care in the Bull-headed Shrike. J Avian Biol 49:e01794. https://doi.org/10.1111/jav.01794
Oberweger K, Goller F (2001) The metabolic cost of birdsong production. J Exp Biol 204:3379–3388
Patel R, Mulder RA, Cardoso GC (2010) What makes vocalisation frequency an unreliable signal of body size in birds? A study on Black Swans. Ethology 116:554–563. https://doi.org/10.1111/j.1439-0310.2010.01769.x
Payne RB (2005) The cuckoos. Oxford University Press, New York
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551. https://doi.org/10.1111/j.1558-5646.1997.tb02441.x
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Accessed 04 Sep 2019
Redpath SM, Appleby MB, Petty SJ (2000) Do male hoots betray parasite loads in tawny owls? J Avian Biol 31:457–462. https://doi.org/10.1034/j.1600-048X.2000.310404.x
Svensson L, Mullarney K, Zetterstrom D, Grant PJ (2010) Collins Bird Guide, 2nd edn. Harper Collins, London
Tryjanowski P, Morelli F (2015) Presence of cuckoo reliably indicates high bird diversity: a case study in a farmland area. Ecol Ind 55:2–58. https://doi.org/10.1016/j.ecolind.2015.03.012
Tryjanowski P, Morelli F, Osiejuk TS, Møller AP (2018) Functional significance of cuckoo Cuculus canorus calls: responses of conspecifics, hosts and non-hosts. PeerJ 6:e5302. https://doi.org/10.7717/peerj.5302
Van Hout AJ-M, Pinxten R, Geens A, Eens M (2012) Non-breeding song rate reflects nutritional condition rather than body condition. PLoS ONE 7:e36547. https://doi.org/10.1371/journal.pone.0036547
Weiss M, Kiefer S, Kipper S (2012) Buzzwords in females’ ears? The use of buzz songs in the communication of Nightingales (Luscinia megarhynchos). PLoS ONE 7:e45057. https://doi.org/10.1371/journal.pone.0045057
Wyllie I (1981) The Cuckoo. Batsford, London
Xia C, Deng Z, Lloyd H, Møller AP, Zhao X, Zhang Y (2019) The function of three main call types in Common Cuckoo. Ethology 125:652–659. https://doi.org/10.1111/eth.12918
Yoo S, Kim H-N, Lee J-W, Yoo J-C (2020) Seasonal and diurnal patterns of population vocal activity in avian brood parasites. Ibis 162:1001–1011. https://doi.org/10.1111/ibi.12741
Yorzinski JL, Vehrencamp SL (2009) The effect of predator type and danger level on the mob calls of the American Crow. Condor 111:159–168. https://doi.org/10.1525/cond.2009.080057
Zsebők S, Moskát C, Bán M (2017) Individually distinctive vocalization in Common Cuckoos (Cuculus canorus). J Ornithol 158:213–222. https://doi.org/10.1007/s10336-016-1376-9
Funding
Open access funding provided by Eötvös Loránd University. Financial support was provided in part by the National Science Foundation IOS #1953226 (to MEH) and the National Research, Development and Innovation Office, Hungary to CM (OTKA #NN118194). MEH was supported by the Hanse-Wissenschaftskolleg (Institute for Advanced Study), Germany, during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Local ethical regulations and agreements were followed for fieldwork. All field work complied with the Hungarian laws, and the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management, Budapest, provided permission for research (permit no. PE/KTF/17190-3/2015).
Additional information
Communicated by T. S. Osiejuk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elek, Z., Bán, M., Fülöp, A. et al. Call rate in Common Cuckoos does not predict body size and responses to conspecific playbacks. J Ornithol 162, 1183–1192 (2021). https://doi.org/10.1007/s10336-021-01909-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01909-6