Abstract
The obligate brood parasitic common cuckoo (Cuculus canorus) is best known for its two-note “cu-coo” call, which is uttered repeatedly by adult males during the breeding season. This call advertises the male’s claim for his territory. A rare, aberrant version (“cu-kee”) was discovered in a population of cuckoos in central Hungary. In a playback experiment, we simulated conspecific territorial intrusions using either aberrant call sequences or normal calls (as control). Cuckoos responded to both calls similarly by approaching the speaker, flying around it several times, and perching on nearby trees. To identify the role of each note of these cuckoo calls, we also played sequences of the first (“cu”) or second (“coo” or “kee”) notes of the calls. Territorial males responded to first notes at similarly high frequencies as to each of the full calls, whereas responses toward either second note type were nearly absent. Thus, the first notes of both typical and aberrant cuckoo calls contain sufficient information to recognize conspecific males and the novel calls did not reduce the efficiency of male-male communication in cuckoos because the aberration occurred in the less functional second note.
Significance statement
Birds use songs and calls to communicate with each other, including advertising their territories to keep competitors away. However, when the acoustic signal is atypical and distorted, the receiver individual may not process it correctly. Common cuckoos recognize a territorial intruder by their well-known “cu-coo” calls. We studied a rare, aberrant version of the common cuckoo call (“cu-kee”), which differed from the normal call in the second note of the two-partite call. However, cuckoos responded similarly to both of the normal and aberrant calls in a playback experiment. When the first or second parts of the different calls were played separately, only the first part of the cuckoo calls was effective in eliciting territorial defence. Consequently, the aberrant second note did not reduce cuckoos’ communication efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vocal communication of animals has two main components: signallers emit acoustic signals, and receivers perceive them and process the information content of the signals (Maynard Smith and Harper 2003; Bradbury and Vehrencamp 2011). Many avian lineages evolved fine-tuned acoustic communication systems and have a great variety of auditory signals for several functions, including songs (Catchpole and Slater 2008) and/or calls (Marler 2004). Oscine birds (Passeriformes), parrots (Psittaciformes), and hummingbirds (Trochilliformes) are able to learn acoustic elements from parents and neighbours, or even imitate different species (Kroodsma and Miller 1982). Other lineages are not vocal-learners and have simpler, innate vocalizations (Brenowitz 1991), which typically show less variation among nearby individuals.
Auditory communication in birds needs effective signals to transmit the information with no or minimal degradation to the receivers (Dooling 1982). However, emitted signals may differ from the received signals (Forrest 1994). For example, habitat structure (Morton 1975; Job et al. 2016), distance (McGregor and Krebs 1984; Mouterde et al. 2014), and anthropogenic traffic noise (Jung et al. 2020) may cause degradation in the structure of the vocal signal and, consequently, also in its information content (Wiley and Richards 1982). Both signallers and receivers show adaptations to environmental conditions to preserve the information of acoustic signals during transmission (Brumm and Naguib 2009). However, degraded sound may nonetheless give some specific but still useful information to the receiver, for example, about distance (McGregor and Krebs 1984).
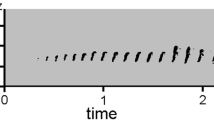
Common cuckoos (Cuculus canorus) are obligate avian brood parasites that lay their eggs in the nests of other bird species, and leave the incubation and rearing of their progeny to these hosts (Payne 2005; Erritzøe et al. 2012). Common cuckoos have a simple acoustic repertoire (Lei et al. 2005); their most famous advertisement call (“cu-coo”) is frequently emitted during the breeding season (Deng et al. 2019a; Yoo et al. 2020). Sometimes, this characteristic call has unique or aberrant features (Møller et al. 2016), and individual cuckoos can be identified based on their call variation; for example, an individual cuckoo was tracked in Germany by its unusual calls for several years (Naumann 1901).
During our long-term research on common cuckoos (Geltsch et al. 2017), we discovered a handful of individuals uttering both normal (“cu-coo”) and aberrant (“cu-kee”) calls (Fig. 1). Whereas we found several individuals with this second type of call in central Hungary, normal and aberrant calls both occurred together in the calling sequences of these same individuals. Here, we investigated whether this rare call type reduced the efficiency of the typical “cu-coo” call in male responses to territorial intrusion using playback experiments. We hypothesised that responses of territorial male cuckoos to aberrant call playbacks would be diminished because these rare signals did not indicate territorial intrusion functionally. Alternatively, the aberrant call might function normally if the aberration affected non-functional parts of the call, which did not contain any or critical signalling content.
Materials and methods
The study was conducted about 50 km south of Budapest, central Hungary, in a 20 km × 30 km area near Apaj (47° 6′ 53.9″ N; 19° 5′ 21.2″ E), where cuckoos parasitize great reed warblers (Acrocephalus arundinaceus) in narrow reed beds along the sides of small irrigation channels (Moskát and Honza 2000). We recorded common cuckoo calls used for our playback study in May between 2017 and 2019. For sound recording, we used a Telinga Universal parabola dish with Rycote Hi Wind Cover, a Sennheiser ME-62 microphone, a K6 powering module, a FEL MX mono preamp, and a Marantz PMD-620 MKII recorder (sampling rate: 48 kHz, 24-bit quality) (Moskát et al. 2017).
To assess the extent of the uniqueness of the aberrant call type, we surveyed the Xeno-Canto (www.xeno-canto.com) database on May 1, 2020; this is a publicly collected and openly available library of avian calls and songs recorded by “citizen science” volunteers (Cooper 2016; Benedetti et al. 2018) and is likely biased by human preference for certain types of bird vocalizations (Blackburn et al. 2014). We also deposited and made available several normal “cu-koo” and three representative “cu-kee” calls (XC562704, XC562706, and XC562707) from our study site in Hungary into Xeno-Canto.
To analyse the acoustic (dis)similarity between the normal and aberrant call types recorded at our study site, we used Raven Pro 1.5 (Cornell Laboratory of Ornithology, Ithaca, USA) which includes a sound correlation feature to compute bioacoustic distance scores between two sound files (e.g. Louder et al. 2019; Hauber et al. 2020). Using this functionality, we generated similarity scores within and between the normal and aberrant calls (n = 10 similarity scores for each of the three types of comparisons) using the spectrogram correlation method.
To assess the functionality of normal vs. aberrant call types, we carried out a playback experiment from May 4 to May 18, 2020. We played cuckoo calls at points on the banks of channels where a male cuckoo’s advertisement call was just heard and the cuckoo was also seen from about 30–40 m (< 50 m). The playback equipment was set up at ca. 1.5 m of height, and the observer stayed hidden behind bushes and trees, but with a good view of the site. Sound files in wav 16-bit format were played by a Lenovo TAB 2 A7 tablet, connected with a 20-m sound cable to a JBL Xtreme (40 W) loudspeaker, and calls were broadcast at 70 dB at 1 m. It was not possible to record the data using blinded methods because our study involved clearly discriminable acoustic playbacks and simultaneously recording vocalizations of focal animals in the field.
For playbacks, we used five different playback files:
-
(i)
Normal advertising calls (“cu-coo”), which were frequently emitted by male common cuckoos in their breeding area (Fig. 1). Cuckoos emit this two-note call repeatedly. Previous playback studies revealed that territorial males approached the loudspeaker when a conspecific “cu-coo” call sequence was played (Moskát et al. 2017, 2018). Because the normal cuckoo call was the unmanipulated reference in this study, we used it as a control.
-
(ii)
Aberrant variants of the advertising calls (“cu-kee”). The first note of the “cu-coo” call was normal, and the second one was an aberrant variant, which sounded unusual to us (Fig. 1). Typically, this aberrant call type occurred in almost every year in our long-term study area, and up to 5% of the individuals were heard in the area in any given year emitting this call type (CM pers. obs.). Interestingly, most individual cuckoos that were detected with aberrant calls also emitted normal calls. This aberrant variant in call sequences was present in such individuals up to 90% of calling (CM pers. obs.).
To assess the functional components of male cuckoos’ conspecific communication signals, we also played single-note components of the full calls as follows:
-
(iii)
The first notes in cu-coo calls (“cu”), taken from normal “cu-coo” calls.
-
(iv)
The second notes in normal cuckoo calls (“coo”).
-
(v)
The aberrant second notes (“kee”).
We filtered out low-frequency noise (ca. below 200 kHz) and normalized for mean amplitude of all stimuli with the Audacity 2.3.3. program.
The structure of the playback files was a sequence of six notes that was repeated three times (altogether 30 s length), following by a 15-s pause. This sequence was repeated three times, without the last pause. Altogether, this playback file lasted 2 min (30-s notes +15-s pause + 30-s notes + 15-s pause and 30-s notes). All playback type files were composed similarly. We constructed 12 different files for each playback type. Because the number of recordings of aberrant calls was limited, we used recordings by an individual cuckoo 1–4 times, but always chose a different sequence of syllables to reduce potential pseudoreplication (Kroodsma 1989). For the same reason, we played every playback file only once, and we choose playback sites distant enough to be in different males’ territories (at least 500 m away). This minimal inter-trial distance was chosen based on a recent study in our population, using GPS telemetry, which revealed that female and male cuckoos are both territorial during their breeding season (Moskát et al. 2019); accordingly, we consider that potentially sampling the same male cuckoo repeatedly was negligible.
During the 2-min playback period, male cuckoos, identified by their frequent sex-specific cu-coo calls, were observed and their movements were dictated into a Tascam DR-05 sound recorder. The distances of male cuckoos were estimated after training with laser rangefinders (Bushnell Yardage Pro 800 and Suaoki 600 m). We also recorded the presence of female cuckoos (if any). In turn, female cuckoos were identified by their sex-specific bubbling calls, which were emitted less often than the male advertising calls (Deng et al. 2019b; Moskát and Hauber 2019; Xia et al. 2019); typically, females were not visible during the playback. In order to avoid the intersexual effects on the responses to our playback, we did not start the experiment at those sites where females and males were interacting, including flying together, or perching on the same branch of a tree. We measured five response variables on male cuckoos during the playbacks (Table 1). Altogether, 37 focal male cuckoos responded to our playbacks by approaching the loudspeaker during the 55 trials encompassing the five categories of call types (responses/playback trials: “cu-coo”: 12/12, “cu-kee”: 12/12, “cu”: 10/11, “coo”: 2/10, “kee”: 1/10). We also observed additional 16 male cuckoos near the speakers at these sites, where positive responses were detected. Male cuckoos frequently uttered their typical “cu-coo” calls during the playback (and also in the pre- and post-playback periods), and we easily observed and followed each of them in the semi-open habitat. In turn, female common cuckoos are more secretive in their behaviours, and never utter the sex-specific “cu-coo” call; therefore, sex-recognition of the responding individuals was not ambivalent. We detected female cuckoos in 16 out of the 55 playback trials at similar frequencies among the playback categories (χ2 = 5.556, df = 4, p = 0.235). No female showed any clear behavioural responses toward our playbacks (e.g. did not approach the speaker, etc.; Table 1). This finding agrees with other published studies that showed no female-specific attract in playbacks of the conspecific males’ “cu-coo” calls (i.e. Moskát et al. 2017; Tryjanowski et al. 2018; Xia et al. 2019).
We used least-square means analysis of variance for the assessment of the biacoustic similarity scores in JMP 12.0 (SAS Institute, Cary, USA). Additional statistical tests were conducted in SPSS package ver. 17.0 (SPSS Corp., Chicago, IL) with alpha = 0.05. Specifically, binary logistic regressions and generalized linear models with the negative binomial model selection were used to assess effects on binary or continuous measures of cuckoos’ behaviour, respectively (Table 1).
Results
Our survey of the Xeno-Canto database revealed the that “cu-kee” type of aberrant call in common cuckoos is not restricted to our study site in central Hungary as we also identified it in the recordings of a single citizen scientist in Germany. His recording contained two “cu-kee” sound files, perhaps taken from the same individual cuckoo (XC244130 and XC246319).
Regarding the biacoustic similarity scores within and between call types, we found consistently high scores within both the “cu-coo” and the “cu-kee” calls, whereas the scores were significantly lower for the comparison between the two call types (F2,27 = 6.9, p = 0.004; Fig. 2).
Acoustic similarity scores within and between “cu-coo” and “cu-kee” calls, as calculated by Raven 1.5 Pro spectrogram correlation function. Each shaded dot represents a unique data point (all n = 10 per each comparison type) and the black dot and shaded whiskers depict the mean + SE. Pairwise post hoc Tukey = comparisons are coo-coo vs. kee-coo: p = 0.0044, kee-kee vs. kee-coo: p = 0.024, and coo-coo vs. kee-kee: p = 0.77
Surprisingly, we observed statistically similar reactions of cuckoos to both of the playback types when we used either full-length normal or aberrant cuckoo calls (Fig. 3, Table S1). They typically approached the speaker, flew around it several times, perched on a nearby tree, and looked toward the source of the playback. In our second set of trials, when only a single note of the full cuckoo calls was used for playback, we obtained markedly different results. On the one hand, playbacks of the first note resulted in statistically similar responses as when the complete, two-note cuckoo calls were played (Fig. 3, Table S1). On the other hand, most of the cuckoos near the playback site showed significantly and drastically less response to the second note types, irrespective of normal or aberrant (Fig. 3, Table S1).
Male common cuckoos’ responses to playback trials with normal (“cu-coo”), aberrant (“cu-kee”), and partial (“cu”, “coo”, or “kee”) common cuckoo calls, when responses are made binary (yes/no). Significance levels were obtained by parameter estimation by the generalized linear model where the dependent variable was closest distance (NS: p > 0.05; *: p < 0.01; see also Table S1)
Binary logistic regression revealed that cuckoos’ behavioural responses (yes/no) depended on the type of the playback file (Table 2a). Another variable component of playbacks, which was the distance of the focal bird from the loudspeaker at the beginning of the playback (i.e. “starting distance”), did not co-vary with response metrics (Table 2a). Generalized linear models revealed that playback type was the only significant predictor (p < 0.001) that affected territorial male cuckoos’ approach to the speaker (“closest distance”; Table 2b, S1). This variable also similarly (p < 0.001) predicted the intensity of responses as indicated by the latency of approach (“latency”; Table 2c, S2).
Discussion
Our results revealed that “cu-kee” aberrant advertisement calls (Fig. 1) were acoustically distinct (Fig. 2) but contained the same communicative information for other male cuckoos as the normal “cu-coo” calls (Fig. 3). We showed that the first note of the cuckoo call functioned as the whole call, containing sufficient information to elicit responses by conspecific males. The second notes, either normal or aberrant, did not contain such information. A previous study on the two parts of the “cu-coo” call revealed that recognizing both parts was necessary for familiarity (Moskát et al. 2018). This suggests that cuckoos may use the aberrant note for familiarity or individual recognition.
In more complex environments and in complex social systems, birds typically use intricate communication systems (Peckre et al. 2019). Although common cuckoos live in diverse habitats, parasitize several host species, and exhibit active social interactions on their breeding ground (Davies 2000), they use a small vocal repertoire for acoustic communication, which is dominated by the males’ two-note “cu-coo” syllables (Tryjanowski et al. 2018; Moskát and Hauber 2019; Xia et al. 2019) over the less frequently heard female-specific bubbling call (Deng et al. 2019b; Moskát and Hauber 2019; Xia et al. 2019). The frequency of male cuckoo calls is low, ca. 500–750 Hz (Zsebök et al. 2017). This allows the “cu-coo” calls to be transmitted at high fidelity, because low-frequency sounds are less affected by small obstacles (e.g. leaves) in the field than high-frequency sounds (Slabbekoorn 2004). Consequently, cuckoo calls can be heard from 2 to 3 km away under good weather conditions (Møller et al. 2016). In turn, male common cuckoos are able to recognize subtle differences among the calls of different individuals (Jung et al. 2014; Li et al. 2017; Zsebök et al. 2017), which makes it possible to discriminate between intruding strangers and familiar neighbours in their territories (Moskát et al. 2017, 2018).
Male common cuckoos are known to vocalize using other, including aberrant forms of calls (Møller et al. 2016), but these are observed and reported rarely. Here, we studied the “cu-kee” call, which occurs in our population at low but consistent frequencies in almost each year of our research during the past 20+ years (Geltsch et al. 2017), whereas it is present in only two of a total of 1152 of common cuckoo recordings in the Xeno-Canto data base (also see above).
Overall, our study aimed to reveal whether and how animals can communicate with distorted or aberrant signals. We probed whether there was a threshold when information content was decipherable for its original function. This and other studies are needed to answer these questions on a wide range of lineages. We discovered that common cuckoos, surprisingly, could use both normal and aberrant acoustic signals for male-male communication effectively. In the future, however, new research should focus on the ontogenetic path and physiological causes that generate and produce aberrant but effective calls in this and other species to explain the biological origins of acoustic diversity in non-vocal-learning lineages.
Data availability
We uploaded representative sound files containing both aberrant and normal common cuckoo calls from our study site in Hungary to Xeno-Canto; codes XC562704 (https://www.xeno-canto.org/562704), XC562706 (https://www.xeno-canto.org/562706), and XC562707 (https://www.xeno-canto.org/562707).
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Benedetti Y, Slezak K, Møller AP, Morelli F, Tryjanowski P (2018) Number of syllables in cuckoo Cuculus canorus calls: a test using a citizen science project. Sci Rep 8:12872. https://doi.org/10.1038/s41598-018-31329-1
Blackburn TM, Su S, Cassey P (2014) A potential metric of the attractiveness of bird song to humans. Ethology 120:305–312. https://doi.org/10.1111/eth.12211
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer, Sunderland
Brenowitz EA (1991) Evolution of the vocal control system in the avian brain. Semin Neurosci 3:339–407. https://doi.org/10.1016/1044-5765(91)90030-R
Brumm H, Naguib M (2009) Environmental acoustics and the evolution of bird song. Adv Stud Behav 40:1–34
Catchpole CK, Slater PJB (2008) Bird songs. Biological themes and variation, 2nd edn. Cambridge University press, Cambridge
Cooper C (2016) Citizen science. Abrams Books, New York
Davies NB (2000) Cuckoos, cowbirds and other cheats. Poyser, London
Deng Z, Lloyd H, Xia C, Li D, Zhang Y (2019a) Within-season decline in call consistency of individual male common cuckoos (Cuculus canorus). J Ornithol 160:317–327. https://doi.org/10.1007/s10336-019-01631-4
Deng Z, Lloyd H, Xia C, Møller AP, Liang W, Zhang Y (2019b) Components of variation in female common cuckoo calls. Behav Process 158:106–112. https://doi.org/10.1016/j.beproc.2018.10.007
Dooling RJ (1982) Auditory perception in birds. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, Production, perception, and design feature of sounds, vol 1. Academic Press, New York, pp 95–130
Erritzøe J, Mann CF, Brammer FP, Fuller RA (2012) Cuckoos of the world, 1st edn. Christopher Helm Publishers Ltd, London
Forrest TG (1994) From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool 34:644–654. https://doi.org/10.1093/icb/34.6.644
Geltsch N, Moskát C, Elek Z, Bán M, Stevens M (2017) Egg spotting pattern in common cuckoos and their great reed warbler hosts: a century perspective. Biol J Linn Soc 121:50–62. https://doi.org/10.1093/biolinnean/blw035
Hauber ME, Taylor DM, Brawn JD (2020) Variable or atypical? Comparing unusual songs of the tufted titmouse with a citizen-science database. J Ornithol (published online). https://doi.org/10.1007/s10336-020-01839-9
Job JR, Kohler SL, Gill SA (2016) Song adjustments by an open habitat bird to anthropogenic noise, urban structure, and vegetation. Behav Ecol 27:1734–1744. https://doi.org/10.1093/beheco/arw105
Jung H, Sherrod A, LeBreux S, Price JM, Freeberg TM (2020) Traffic noise and responses to a simulated approaching avian predator in mixed-species flocks of chickadees, titmice, and nuthatches. Ethology 126:620–626. https://doi.org/10.1111/eth.13013
Jung WJ, Lee JW, Yoo JC (2014) “Cu-coo”: can you recognize my stepparents? A study of host-specific male call divergence in the common cuckoo. PLoS One 9:e90468. https://doi.org/10.1371/journal.pone.0090468
Kroodsma DE (1989) Suggested experimental designs for song playbacks. Anim Behav 37:600–609. https://doi.org/10.1016/0003-3472(89)90039-0
Kroodsma DE, Miller EH (1982) Acoustic communication in birds, vol. 2: Song learning and its consequence. Academic Press, New York
Lei F-M, Zhao H-F, Wang A-Z, Yin Z-H, Payne RB (2005) Vocalizations of the common cuckoo Cuculus canorus in China. Acta Zool Sin 51:31–37
Li Y, Xia C, Lloyd H, Li D, Zhang Y (2017) Identification of vocal individuality in male cuckoos using different analytical techniques. Avian Res 8:21. https://doi.org/10.1186/s40657-017-0079-0
Louder MIM, Balakrishnan CN, Louder ANA, Driver RJ, London SE, Hauber ME (2019) An acoustic password enhances auditory learning in juvenile brood parasitic cowbirds. Curr Biol 29:4045–4051. https://doi.org/10.1016/j.cub.2019.09.04
Marler P (2004) Bird calls: a cornucopia for communication. In: Marler P, Slabbekoorn H (eds) Nature’s music. The science of birdsong. Elsevier Science, San Diego, pp 132–177
Maynard Smith J and Harper D (2003) Animal signals. Oxford University Press, Oxford
McGregor PK, Krebs JR (1984) Sound degradation as a distance cue in great tit (Parus major) song. Behav Ecol Sociobiol 16:49–56. https://doi.org/10.1007/BF00293103
Møller AP, Morelli F, Mousseau TA, Tryjanowski P (2016) The number of syllables in Chernobyl cuckoo calls reliably indicate habitat, soil and radiation levels. Ecol Indic 66:592–597. https://doi.org/10.1016/j.ecolind.2016.02.037
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34. https://doi.org/10.1086/282971
Moskát C, Hauber ME (2019) Sex-specific responses to simulated territorial intrusions in the common cuckoo: a dual function of female acoustic signaling. Behav Ecol Sociobiol 73:60. https://doi.org/10.1007/s00265-019-2665-0
Moskát C, Honza M (2000) Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography 23:335–341. https://doi.org/10.1111/j.1600-0587.2000.tb00289.x
Moskát C, Bán M, Fülöp A, Bereczki J, Hauber ME (2019) Bimodal habitat use in brood parasitic common cuckoos (Cuculus canorus) revealed by GPS telemetry. Auk 136:1–12. https://doi.org/10.1093/auk/uky019
Moskát C, Elek Z, Bán M, Geltsch N, Hauber ME (2017) Can common cuckoos discriminate between neighbours and strangers by their calls? Anim Behav 126:253–260. https://doi.org/10.1016/j.anbehav.2017.02.013
Moskát C, Hauber ME, Bán M, Fülöp A, Geltsch N, Marton A, Elek Z (2018) Are both notes of the common cuckoo's call necessary for familiarity recognition? Behav Process 157:685–690. https://doi.org/10.1016/j.beproc.2018.03.017
Mouterde SC, Theunissen FE, Elie JE, Vignal C, Mathevon N (2014) Acoustic communication and sound degradation: how do the individual signatures of male and female zebra finch calls transmit over distance? PLoS One 9:e102842. https://doi.org/10.1371/journal.pone.0102842
Naumann JA (1901) Naturgeschichte der Vögel Mitteleuropas, vol 4. Gera-Untermhaus and Köhler, Gera
Payne RB (2005) The cuckoos. Oxford University Press, New York
Peckre L, Kappeler PM, Fichtel C (2019) Clarifying and expanding the social complexity hypothesis for communicative complexity. Behav Ecol Sociobiol 73:11. https://doi.org/10.1007/s00265-018-2605-4
Slabbekoorn H (2004) Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H (eds) Nature’s music. The science of birdsong. Elsevier Science, San Diego, pp 168–205
Tryjanowski P, Morelli F, Osiejuk TS, Møller AP (2018) Functional significance of cuckoo Cuculus canorus calls: responses of conspecifics, hosts and non-hosts. PeerJ 6:e5302. https://doi.org/10.7717/peerj.5302
Xia C, Deng Z, Lloyd H, Møller AP, Zhao X, Zhang Y (2019) The function of three main call types in common cuckoo. Ethology 125:652–659. https://doi.org/10.1111/eth.12918
Yoo S, Kim H-N, Lee J-W, Yoo J-C (2020) Seasonal and diurnal patterns of population vocal activity in avian brood parasites. Ibis 162:1001–1011. https://doi.org/10.1111/ibi.12741
Wiley RH, Richards DG (1982) Adaptations for acoustic communication in birds. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, Production, perception, and design feature of sounds, vol 1. Academic Press, New York, pp 131–182
Zsebök S, Moskát C, Bán M (2017) Individually distinctive vocalization in common cuckoos (Cuculus canorus). J Ornithol 158:213–222. https://doi.org/10.1007/s10336-016-1376-9
Acknowledgements
We are indebted to Hannah Scharf for help with a figure and to Thomas Gavin for linguistic assistance. We also thank the referees and the editor of this journal for their constructive comments.
Funding
Open Access funding provided by Hungarian Natural History Museum. The study was supported by the National Research, Development and Innovation Office, Hungary to CM (OTKA #NN118194), with additional funding from the USA National Science Foundation (IOS #1456524) and the National Geographic Society (NGS-60453R-19) to MEH.
Author information
Authors and Affiliations
Contributions
CM and MEH conceived and designed the study, CM conducted fieldwork, DMT conducted the bioacoustics analyses, CM and MEH conducted the statistical analyses, and CM wrote the first draft of the manuscript with contributions from MEH. All authors edited and approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Local ethical regulations and agreements were followed for fieldwork. All work complied with the Hungarian laws, and the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management, Budapest, provided permission for research (permit no. PE/KTF/17190-3/2015).
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by M. Soler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 195 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moskát, C., Taylor, D.M. & Hauber, M.E. Effective conspecific communication with aberrant calls in the common cuckoo (Cuculus canorus). Behav Ecol Sociobiol 75, 7 (2021). https://doi.org/10.1007/s00265-020-02946-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02946-6