Abstract
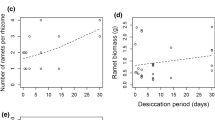
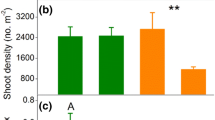
To confirm the role of winter buds and rhizome morphology on the winter survival and subsequent growth of common reed (Phragmites australis), rhizome fragments of varying sizes with and without winter buds from three different P. australis populations were transplanted to small pots in a greenhouse and wintered by exposing them to a dry atmospheric condition. Survival ratios of the rhizome fragments with winter buds were much greater than those without winter buds regardless of population type, supporting that winter buds play a role as the insulator of rhizome fragments from dry atmosphere during winter season. Most growth characteristics of surviving P. australis from the rhizome fragments were similar to those in natural wetlands, indicating preserved growth characteristics through the rhizomes, which is an asexual organ. The average number of rhizome fragment nodes showed negative relationships with the number of blades per culm (P < .05) and basal culm diameter (P < .05) of surviving P. australis. In contrast, the diameter of planted rhizome fragments had significant positive relationships with the final shoot height (P < .05), above-ground dry weight (P < .05), root DW (P < .01), and total DW (P < .05). Rhizome volume also showed a similar correlation with the rhizome diameter. Thicker and larger rhizome fragments particularly with winter buds, indicating the young age of rhizome, seemed likely to guarantee greater survival and higher subsequent shoot growth and biomass production as well.



Similar content being viewed by others
References
Armstrong J, Armstrong W, van der Putten WH (1996) Phragmites die-back: bud and root death, blockages within the aeration and vascular systems and the possible role of phytotoxins. N Phytol 133:399–414
Asaeda T, Karunaratne S (2000) Dynamic modeling of the growth of Phragmites australis: model description. Aquat Bot 67:301–318
Asaeda T, Nam LH (2002) Effects of rhizome age on the decomposition rate of Phragmites australis rhizomes. Hydrobiologia 485:205–208
Asaeda T, Manatunge J, Roberts J, Hai DN (2006) Seasonal dynamics of resource translocation between the aboveground and age-specific rhizome segments of Phragmites australis. Environ Exp Bot 57:9–18
Bart D, Hartman JM (2003) The role of large rhizome dispersal and low salinity windows in the establishment of common reed, Phragmites australis, in salt marshes: new links to human activities. Estuaries 26:436–443
Chambers RM, Meyerson LA, Salltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273
Chambers RM, Osgood DT, Bart DJ, Montalto F (2003) Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26:398–406
Čížková H, Lukavská J (1999) Rhizome age structure of three populations of Phragmites australis (Cav.) Trin. Ex Steud.: biomass and mineral nutrient concentrations. Folia Geobot 34:209–220
Fiala K (1976) Underground organs of Phragmites australis, their growth, biomass and net production. Folia Geobot 11:225–259
Haslam SM (1969) The development and emergence of buds in Phragmites communis Trin. Ann Bot 33:289–301
Hong M-G, Kim JG (2012) Growth characteristics of cutting culms sectioned at different positions from three reed populations. J Korean Environ Res Technol 15:53–62 (in Korean with English abstract)
Hong M-G, Kim JG (2013) Cutting efficiency using Phragmites australis culms according to content and timing of indole-acetic acid treatment. J Wetl Res 15:35–41 (in Korean with English abstract)
Howard RJ, Rafferty RS (2006) Clonal variation in response to salinity and flooding stress in four marsh macrophytes of the northern gulf of Mexico, USA. Environ Exp Bot 56:301–313
Karunaratne S, Asaeda T, Toyooka S (2004) Colour-based estimation of rhizome age in Phragmites australis. Wetl Ecol Manag 12:353–363
Kausch AP, Seago JL, Marsh LC (1981) Changes in starch distribution in the overwintering organs of Typha latifolia (Typhaceae). Am J Bot 68:877–880
Klimeš L (2000) Phragmites australis at an extreme altitude: rhizome architecture and its modeling. Folia Geobot 35:403–417
Klimeš L, Klimešová J, Čižková H (1999) Carbohydrate storage in rhizomes of Phragmites australis: the effects of altitude and rhizome age. Aquat Bot 64:105–110
Lissner J, Schierup H–H (1997) Effects of salinity on the growth of Phragmites australis. Aquat Bot 55:247–260
McNaughton SJ (1975) r- and K-selection in Typha. Am Nat 109:251–261
Moore M, Romano SP, Cook T (2010) Synthesis of upper Mississippi river system submerged and emergent aquatic vegetation: past, present, and future. Hydrobiologia 640:103–114
Park J, Hong M-G, Kim JG (2013) Relationship between early development of plant community and environmental condition in abandoned paddy terraces at mountainous valleys in Korea. J Ecol Environ 36:131–140
Rejmánková E (2011) The role of macrophytes in wetland ecosystems. J Ecol Environ 34:333–344
Schimming WK, Messersmith CG (1988) Freezing resistance of overwintering buds of four perennial weeds. Weed Sci 36:568–573
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv Biol 18:1424–1434
Soetaert K, Hoffmann M, Meire P, Starink M, van Oevelen D, van Regenmortel S, Cox T (2004) Modeling growth and carbon allocation in two reed beds (Phragmites australis) in the Scheldt estuary. Aquat Bot 79:211–234
Squires L, van der Valk AG (1992) Water-depth tolerance of the dominant emergent macrophytes of the Delta Marsh Manitoba. Can J Bot 70:1860–1867
Thompson DJ, Shay JM (1985) The effects of fire on Phragmites australis in the Delta Marsh, Manitoba. Can J Bot 63:1864–1869
Thompson DJ, Shay JM (1989) First-year response of a Phragmites marsh community to seasonal burning. Can J Bot 67:1448–1455
Zhou J, Wang D (2012) Effects of turion size and water depth on germination and growth of an aquatic plant (Myriophyllum oguraense Miki subsp. yangtzense). J Freshw Ecol 27:287–294
Acknowledgments
We thank Jonathan Shin and Seung Cheol Ko for field and mesocosm works. This study was supported by the Center for Aquatic Ecosystem Restoration (CAER) of the Eco-STAR project (EW33-08-12) and Eco-innovation project (416-111-010) from the Ministry of Environment, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, M.G., Kim, J.G. Role and effects of winter buds and rhizome morphology on the survival and growth of common reed (Phragmites australis). Paddy Water Environ 12 (Suppl 1), 203–209 (2014). https://doi.org/10.1007/s10333-014-0445-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-014-0445-z