Abstract
Habitat destruction and over-hunting are increasingly threatening the arboreal primates of Central Africa. To establish effective conservation strategies, accurate assessments of primate density, abundance, and spatial distribution are required. To date, the method of choice for primate density estimation is line transect distance sampling. However, primates fleeing human observers violate methodological assumptions, biasing the accuracy of resulting estimates. In this study, we used line transect distance sampling to study five primate species along 378 km of transects in Salonga National Park, Democratic Republic of the Congo. We tested the effect of different levels of survey-inherent disturbance (i.e., cutting) on the number of observed (i) primate groups, and (ii) individuals within groups, by counting groups at three different time lags after disturbance of the transect, (i) a minimum of 3 h, (ii) 24 h, (iii) a minimum of 3 days. We found that survey-inherent disturbance led to underestimated densities, affecting both the number of encountered groups and of observed individuals. However, the response varied between species due to species-specific ecological and behavioral features. Piliocolobus tholloni and Colobus angolenis resumed an unaltered behavior only 24 h after disturbance, while Lophocebus aterrimus, Cercopithecus ascanius, and Cercopithecus wolfi required a minimum of 10 days. To minimize bias in density estimates, future surveys using line transect distance sampling should be designed considering survey-inherent disturbance. We recommend evaluating the factors driving primate response, including habitat type, niche occupation, and hunting pressure, peculiar to the survey-specific area and primate community under study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effective conservation of wild animal populations requires accurate estimates of their distribution, density, and abundance, information critical to the assessment of population status and temporal trends (Nichols and Williams 2006). Consequently, for obtaining unbiased estimates, reliable field methods are equally crucial to correctly informing conservation strategies.
This is particularly true for taxa of high conservation importance, such as primates (Chapman et al. 2020). There is a rich body of literature describing methods and best practices for great ape density estimates (Kühl et al. 2008), iconic species suffering catastrophic population declines (Carvalho et al. 2021; Junker et al. 2012; Kühl et al. 2017; Plumptre et al. 2016). However, the same does not apply to the majority of monkey species. Unlike great apes, for which population density can be estimated making use of their habit to build sleeping platforms called “nests” (Tutin and Fernandez 1984), other primate species do not leave obvious signs of their presence and must be monitored by direct observation or by their vocalizations (Plumptre et al. 2013). Nevertheless, many of these species are also decreasing as a result of habitat destruction (Cavada et al. 2019; Remis and Jost Robinson 2012) and over-hunting (Kümpel et al. 2008; Linder and Oates 2011; Peres 1999; Rosenbaum et al. 1998). In Central Africa, primate habitat loss is accelerating due to (1) small-scale slash-and-burn agriculture of increasing rural population (Tyukavina et al. 2018), as well as (2) widespread extraction of natural resources like minerals and timber driven by increasing global demand (Abernethy et al., 2016). The problem is exacerbated by hunting pressure. In fact, primates are among the most affected species, being targeted by both commercial (Bachand et al., 2015; van Vliet et al. 2012) and subsistence hunters (Fa et al. 2016).
The debate on best practices for the assessment of arboreal primate population status is still ongoing. Primates inhabit highly diverse habitats, spanning open savannahs, rainforest, and mountain ranges, and differ significantly in terms of ecology and behavior. As a result, the survey methods suitable for a species in a given habitat, may not provide accurate or precise density estimates in other contexts. To overcome the complexity of surveying arboreal primates in the wild, depending on the habitat and species of interest, recent methods suggested the application of acoustic playback (Gestich et al. 2017), passive acoustic monitoring (Kalan et al. 2015), camera traps (Bessone et al. 2020; Moore et al. 2020), and drones (Semel et al. 2020; Spaan et al. 2019). However, these novel techniques are still under development and cannot be applied to all species (e.g., acoustic methods can only be used for highly vocal species), leaving line transect distance sampling (LTDS) as the method of choice for surveying primates (Buckland et al. 2001; Plumptre et al. 2013). LTDS consists of linear sample units, called transects, paths walked by trained observers to count primates in vision. Being based on direct observations, LTDS has the advantage of being applicable to any species. As each observed primate is recorded along with its perpendicular distance from the transect, LTDS allows estimating density by modeling the probability of observing a monkey as a function of distance. In short, the further a monkey is from the transect, the lower the probability of spotting it. By modeling detection probability, the application of distance sampling provides unbiased estimates of the true population density in the study area, conditional on sufficient survey effort and the deployment of an adequate number of transects (n > 20, i.e., replication) placed randomly throughout the study area (i.e., randomization), as well as the fulfilment of certain assumptions (Buckland et al. 2001).
In the past, however, the application of LTDS to primates raised concerns (Chapman et al. 2010; Hassel-Finnegan et al. 2008; Marshall et al. 2008). Some studies reported large overestimates of the true primate density (Chapman et al. 2010; Defler and Pintor 1985; Hassel-Finnegan et al. 2008), while others showed the opposite (Cavada et al. 2017a, b; Skorupa 1987). Mostly, the reported biases were based on poor survey designs, with studies lacking adequate effort, replication, and randomization (Buckland et al. 2010a). However, even when surveys are carefully designed, surveying primates remains challenging. In order to obtain reliable density estimates, three main issues, along with their related assumptions, must be thoroughly considered during data collection and analysis.
1) Group size. As social mammals, most arboreal primates range and feed in groups up to several dozens of individuals. Because measuring distances to each individual within a group is, albeit preferable, rarely feasible, LTDS usually considers observation of groups rather than individual monkeys (Marshall et al. 2008; Plumptre and Cox 2006). Abundance in the area is then estimated as a function of group size, which must be accurately recorded (assumption 1). In the field, however, primate group size is difficult to assess, particularly in tropical forest where group members are scattered and hidden in the canopy (Araldi et al. 2014; Ferrari et al. 2010). While inaccurate estimation of group size far from the transect line is not expected to cause bias, it is crucial that at least the size of groups close to the line is accurately estimated (Buckland et al. 2010a).
2) Group spread. To correctly estimate the detection probability, LTDS requires the measurement of the perpendicular distance of each observed group to the transect line. Perpendicular distances need to be measured to the group center, and it is assumed that groups with their center approximately on the transect line, are detected with certainty (assumption 2). Furthermore, LTDS assumes that perpendicular distances are measured accurately (assumption 3). Therefore, to locate the group center, observers must first define the spread of the group. As this is not a trivial task (Buckland et al. 2010a, b; Chapman et al. 2010; Marshall et al. 2008), methods improving standard applications have been recently proposed (Cavada et al. 2017a, b).
3) Reactivity to the observer. Finally, LTDS requires that groups must be detected before any response to the observer, i.e., before they flee (assumption 4). Violation of assumption 4 would bias the estimated density by prohibiting observers from correctly detecting groups, eventually constraining the accuracy of the detection function. In tropical forests, the issue is exacerbated by the thick understorey causing disturbance when walking, but more importantly requiring observers to cut their line transects. In addition, primates may react by avoiding areas previously visited by the researchers, e.g., where transects have been cut recently. There are different practical suggestions to minimize disturbance such as (a) reducing the noise of cutting by using secateurs rather than machetes (Buckland et al. 2010a); (b) cutting the transect a few days before the actual survey (Plumptre et al. 2013), e.g., 7 days (Araldi et al. 2014; Hofner et al. 2020). However, to our knowledge, studies investigating the effect of disturbance on counts, including recommendations as of the time-lag needed between transect cutting and actual count, are absent.
In this study, we investigated the effect of primate reactivity to the observer. We tested the effect of the time between the disturbance “transect cutting” using machetes, and the actual count on (a) encounter rates (ER), i.e., number of groups per km; (b) observed group size (GS), i.e., number of individual primates within a group; (c) estimated densities (d), i.e., number of individuals per km2; and (e) species-specific differences in (a), (b), and (c).
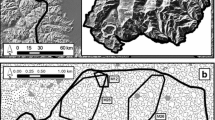
To do so, we applied LTDS in Salonga National Park (SNP), Democratic Republic of the Congo (DRC), the largest protected forest area of the African continent, to five primate species: Tshuapa red colobus (Piliocolobus tholloni), Angolan colobus (Colobus angolensis), Black mangabey (Lophocebus aterrimus), red-tailed monkey (Cercopithecus ascanius whitesidei), and Wolf’s monkey (Cercopithecus wolfi). The population of these species are considered decreasing in the wild, with four of them being classified as vulnerable or endangered: P. tholloni (VU), C. angolensis (VU), L. aterrimus (VU), C. crysogaster (EN), C. wolfi (NT) (IUCN 2020).
Methods
Study area
Salonga National Park (36,000 km2) is an UNESCO World Heritage Site situated in DRC. It is formed by two blocks, North and South, separated by an inhabited corridor (9000 km2). We investigated the block South (17,127 km2), composed 99% of primary lowland mixed forest, 1% of savannahs, regenerating forest, cultivation, marshes and water bodies (Bessone et al. 2020). Nine diurnal primate species are known to be present in the park. In addition to those mentioned above, SNP harbors the endangered Golden bellied mangabey (Cercocebus crysogaster), Allen’s Swamp monkey (Allenopithecus nigoviridis), De Brazza’s monkey (Cercopithecus neglectus), and a great ape, the bonobo (Pan paniscus).
Data collection
General design
LTDS data were collected between September 2016 and May 2018 as part of a comprehensive biodiversity inventory (PNS-Survey©). The survey consisted of 405 transects and was designed in Distance 6.0. (Thomas et al., 2010), with transects starting from a random origin and then placed systematically in the study area. Each transect was 1 km long, spaced from other transects by 6 km in both the east–west and north–south directions (Fig. 1). This design allowed us to (1) obtain a uniform coverage of the study area (Thomas et al., 2010) and (2) survey one transect and reach the next one in a single day. As in our study, five monitoring teams surveyed 6–8 transects per month (i.e., one survey block per month) independently in different areas, the latter was logistically important, as it allowed us to complete a survey block within the planned timeframe.
Each transect was walked 3–4 times. The number of passages (i.e., repeated transect walks), was a trade-off between (1) the need to investigate how survey-inherent disturbance affected primate counts at different times after the first disturbance event, and (2) the feasibility of the study in terms of logistics and time.
To minimize disturbance, we walked in teams of four members only, without carrying loads except for the equipment needed for observing wildlife, measuring perpendicular distances, and recording data. To increase the probability of spotting all primate groups present on the transect, we walked the transects silently and no faster than 0.6 km per hour, minimizing the chances of startling the groups. We also did not walk the transects in the rain, when primates are less active, and did not mark the starting and ending point of the transect in any way visible to the primates. In subsequent passages, we recognized the transect starting point by using its GPS location (marked during the first passage), while the transect line was identified by the presence of (1) a Topofil® thread and (2) vegetation cuts left during the previous passage.
During passage 1 (P1), each transect was opened. To minimize disturbance, we used secateurs whenever possible, although machetes were allowed to be used when required by the thickness of the understory. During P1, one observer was tasked with cutting the transect, while the other three were only required to observe wildlife and wildlife’ signs. We assumed P1 to cause the highest disturbance level and focused on counting indirect signs of sympatric wildlife such as droppings, nests, tracks, and human activity signs rather than on directly observing primates. If a monkey group was observed during P1, species and group size were recorded, but not its distance to the transect. Thus, P1 was not used for the estimation of primate density. P1 was performed in the morning, not before 7:00 am.
Passage 2 (P2) was walked on the same day of P1, and it was the first passage where we recorded perpendicular distance to observed primate groups. It was performed in the afternoon, not before 3:00 pm, as primates are mostly active in the morning and in the late afternoon. In addition, to allow a few hours for the monkeys to recover from potential disturbance, minimum time-lag to completion of P1 was 3 h (N’Goran et al., 2016. By that, we assumed P2 to have the second-highest level of disturbance.
Passage 3 (P3) was performed the day after P1 and P2 and also focused on primate counts. It had to occur in the morning (from 7:00 am) but was postponed to the afternoon (after 3:00 pm) in case of morning rain. We considered P3 to allow even more time for the monkeys to readjust, and thus to have the third-highest level of disturbance. After P3 was completed, the monitoring teams immediately moved to the next transect, in order to reach it before dusk.
Passage 4 (P4) also focused on primate counts but was performed a minimum of 3 days after P1 was completed, either at 7:00 am or after 3:00 pm. P4 had the purpose to assess the influence of disturbance on the detection of monkey groups. For logistical reasons, this passage was not aimed to be conducted on all (n = 405) transects but on a selection of at least 10% (n = 41). To achieve this percentage, we selected one transect per survey block consisting of 6–8 transects each. We assumed P4 to have the lowest level of disturbance.
When we heard a primate group vocalizing, we recorded the point on the transect from where we first heard the group and estimated its distance to the transect and identified the species from its vocalization. Here, as we could not directly observe them, we were unable to identify poly-specific groups with certainty and thus considered each species as mono-specific group. The estimated distances to the transect were then recorded as (a) “close”, if assumed to be closer that 100 m; (b) “far” if estimated being between 100 and 500 m; and (c) “very far” if further than 500 m.
When we spotted a primate group, all observers left the transect line to determine group (a) size, (b) spread/center, and (c) perpendicular distance to the transect line.
Determining group size
We recorded two different values of group size: (1) observed, i.e., how many individual primates were directly spotted; (2) estimated, i.e., how many individual primates were inferred to be present by adding individuals heard but not seen to those observed. Each team member recorded observed group size (1) resulting in four independent counts. In case of poly-specific groups, we counted the number of individuals of each species. For each species, we retained the highest value observed. The same was done for the estimated group size (2). Here, to control for extreme estimated values and/or the risk of consistent upward bias due to the involvement of different observers, we retained the average of the estimated group sizes (n = 4) rather than the highest value.
Estimating group spread
When determining group spread, we focused on observed individuals only (N’Goran et al., 2016). If the group was at one side of the transect only (1), we estimated group spread by determining the position of the closest and furthest individuals observed. However, if the group was at both sides of the transect (2), we estimated group spread by detecting the position of the furthest individuals observed at each side. In both cases, we defined the group center as the midpoint between the two individuals. In case of poly-specific groups, we used the same point for each species.
Measuring perpendicular distances
When we defined the location of the group center, we measured (measuring tape) the perpendicular distance from the group center (in centimeters) to the transect line (marked by a Topofil® thread) following the principles of distance sampling (Buckland et al., 2010a). Finally, we marked the point on the transect perpendicular to a group’s center using a handheld GPS (Garmin 64S) and recorded all additional information on a smartphone (Samsung Galaxy XCover 3) using the Cybertracker software (ver. 3.435).
All team members were trained in LTDS techniques, including (1) compass/GPS navigation; (2) transect cutting and walking, (3) primate group size, spread and perpendicular distance estimation, (4) data recording using the Cybertracker software (N’Goran et al. 2016), during theoretical and practical workshops conducted (1) August 2016 (Monkoto, Tshuapa, DRC) and (2) September 2017 (Mundja, Mai Ndombe and Anga, Kasai, DRC) (Bessone et al. 2019).
Data analysis
We tested for specific differences in encounter rates, i.e., group observed or heard per kilometer, between passages by comparing the average encounter rate (ER) of (a) all species taken together and (b) each primate species. To do so, we modeled ER in each transect i
where ηr is the mean encounter rate in passage r, and φ is the overdispersion parameter for passage r. The negative binomial distribution fitted our dataset, as 46% of transects had no observations (i.e., ER = 0) and a few transects had many observed groups.
Similarly, we checked for differences in species-specific observed group size, i.e., number of individuals per group, between passages by comparing the mean observed group size (GS) in each transect i
where μris the mean group size and σr is the standard deviation in passage r. Here, the lognormal distribution was used to fit a dataset of positive values only. In this analysis, we only considered transects where we had at least one observed group (i.e., GS > 0) and excluded observations made in P1. By that, we avoided bias due to a possible lack of attention in assessing GS in P1, where monkey counts were not the focus.
We coded our models using the package RStan ver. 2.26.11 (Stan Development Team, 2020) in R 4.1.1 (R Core Team, 2021). For each model, we ran four chains of 2000 iterations (1000 warm-up).
We estimated primate density using Distance 7.3 for Windows (Thomas et al., 2010). We first fitted a single detection function for the aggregated analysis of all species together, and then species-specific detection functions to estimate specific densities per passage. For each analysis, we first right-truncated the data following the visual inspection of the histogram of observed distances. Then, we compared models derived from all possible combinations of key function (i.e., half normal; hazard rate; uniform), series expansion (i.e., cosine; simple polynomial) and adjustment terms (i.e., from 0 to 3), selecting the best-fitting model according to lowest Akaike information criterion (AIC). We considered group size in our analyses by estimating an average group size for each species. To do so, we regressed the natural logarithm of group size against distance if the significance of the regression was significant at an alpha level of 0.1. By that, we aimed to reduce the bias induced by smaller groups being missed at larger distances more often than large groups. However, if the regression was not significant, the average group size was equated to the observed average size, i.e., no regression was used (Thomas et al., 2010). As we wanted to highlight differences in estimated density between passages for different species, we performed density analyses for all datasets for which it was possible to obtain a good fitting of the detection function.
However, in order to provide the most reliable density estimates, we calculated a “corrected density” by (1) only considering the passages providing the highest encounter rates; (2) correcting the resulting estimates of group density by the highest average estimated group size obtained within passages. In practice, we did not consider passages providing significantly lower encounter rates when calculating the corrected density (Table 1). Similarly, as we assumed that the highest estimated group size between passages was closer to the real group size, we multiplied the estimated number of primate groups (for each species) obtained by the largest estimated group size returned from passage specific Distance analyses (Table 2).
Results
Due to logistical constraints (i.e., accessibility, safety), we conducted primate counts on 378 transects (out of 405 planned), including repeated passages on the same transects (n = 1158). Due to rain in the afternoon, P2 was conducted on 358 transect only, while the presence of armed poachers (n = 1) and the flooding of the transect area after heavy rain (n = 6) prevented conducting P3 on seven transects, which was thus only conducted on 371 transects. The resulting total effort was 1147.28 km of transects. Of these, 66% (n = 761) were walked in the morning, while the remaining 34% (n = 397) were walked in the afternoon (Table 1). P4 was conducted on 13% of surveyed transects (n = 51), 10.33 days on average (SD = 5.88) after the transect cutting (P1). Here, 53% (n = 29) of transects were walked in the morning and 47% (n = 22) in the afternoon.
Encounter rates (ER)
We encountered a total of 1153 primate groups resulting in an average encounter rate of one group per kilometer (Table 2), including re-sightings of the same group (as same transects were surveyed multiple times). Of these, 1085 groups were mono-specific, and 68 were poly-specific, including two (n = 57) or three (n = 11) different species. We observed fewer poly-specific groups per kilometer in P1. Encounter rates increased with reduced disturbance, with P4 showing three times higher encounter rates of mixed groups than P1 (Table 1). The average observed group size consisted of 8.89 (SD = 8.87) individuals, the average estimated group size of 12.34 individuals (SD = 12.14). Estimated groups sizes were 39% larger than observed ones.
In addition, the total number of encounters included 525 groups only heard vocalizing (i.e., not observed), 97% of which were estimated being within 500 m from the transect line. Here, P2 showed a larger proportion of “far” vocalizations (54%) than other passages (P1 = 44%; P3 = 39%; P4 = 24%) (Table 1). There was a trend to differences being significant between P2 and P3 (X2 = 5.678, p = 0.055) as well as P2 and P4 (X2 = 6.524, p = 0.064) when performing pairwise X2 tests between passages. All other comparisons were not significant (p > 0.1).
When testing for differences in encounter rates (ER) between passages, for most species rates consistently increased with reduction of disturbance, with the highest ER found in P3 and P4 (Fig. 2). This did not apply to the black mangabey L. aterrimus, for which we observed the highest ER in P1 (highest disturbance), followed by P4 (lowest disturbance). The black mangabey was also the species with the highest proportion of heard groups during P1, with 76% of encounters being vocalizations rather than direct observations. This percentage decreased to 32% in P4 (Supporting Table 1).
Differences in encounter rates between passages and species. a Average encounter rate for each passage (P1 = red; P2 = orange; P3 = yellow; P4 = green) by species separated (rows 1 to 5) and together (bottom row); b posterior distribution of pairwise contrasts of average encounter rates between passages for each species
Group size (GS)
The analysis of the differences in group size between passages revealed a similar pattern, with observed group size being higher in P4 with the lowest disturbance. Here, the main exception was represented by the red colobus P. tholloni, showing group size consistent between passages (Fig. 3). Similar results were obtained with estimated group size (Supporting Fig. 1), with the two measures being highly correlated (Spearman rank correlation test: rho = 0.94, p < 0.001).
Differences in observed group size between passages and species. a Average group size observed in each passage (P2 = orange; P3 = yellow; P4 = green) by species (rows 2 to 6) and all species together (top row); b Posterior distribution of pairwise contrasts of average observed group size between passages for each species
Density estimates (D)
Calculated densities increased considerably with a reduction in disturbance (Table 2), with P2 returning the lowest estimates for all species. The limited number of observed groups in P4 did not allow to fit a detection function and calculate a density value for three species: C. tholloni (n = 6), C. angolensis (n = 2) and C. wolfi (n = 7). In addition, due to the small number of transects and observed groups in P4 (Supporting Table 1), confidence intervals (CI) associated with densities the two species with sufficient observations, L. aterrimus and C. ascanius, were very large. Here, the 95% CI obtained in P4 completely overlapped the one obtained in P3. However, the black mangabey was again an exception, showing estimated densities consistent between passages (Table 2). The use of estimated group size consistently increased estimated densities for all species and across all passages by 36% on average (min = 20%, max = 58%) (Table 2).
Following the results above, we also calculated the corrected estimate for each species. Here, we included all observations from P3 and P4, showing consistent ER, discarding those obtained in P2. Then we corrected for group size by using the largest average estimated size obtained. Consequently, we used estimated size observed in P3 for P. tholloni and C. angolensis, and corrected by estimated size obtained in P4 for L. aterrimus, C. ascanius and C. wolfi (Table 2).
By that, we obtained densities of (i) 90 ind/km2 (95% CI = 61–134) for L. aterrimus; (ii) 99 ind/km2 (61–161) for P. tholloni; (iii) 7 ind/km2 (4–12) for C. angolensis; (iv) 62 ind/km2 (45–85) for C. ascanius; and (v) 43 ind/km2 (26–70) for C. wolfi, similar to other sites (Table 3). We provide a detailed description of these analyses, including sample size, selected models, and plots of fitted detection functions of the corrected analyses, in Supporting Table 2.
Discussion
Our study used 1147.28 km of 1-km line transects evenly spread over 17,127 km2 of pristine lowland rainforest, to investigate how survey-inherent disturbance affected primate detectability, hence the accuracy of resulting estimates of density and abundance. We found that survey-inherent disturbance had a negative effect on both encounter rate (ER) and observed group size (GS), leading to underestimated densities when applying LTDS. This is an important finding with far-reaching consequences, which, to our knowledge, has received only little attention by both practitioners and researchers.
Encounter rates (ER)
The number of encountered groups per km of transect (ER) was lowest the day the transect was opened (P1), remaining consistent for a few hours (P2). The ER increased substantially in P3, only 24 h after the highest disturbance in P1, and remained stable in P4, occurring a minimum of 72 h after P1 (Fig. 2). By that, our results suggest that 24 h were sufficient for the primate groups to regain their usual routine and area of activity after disturbance. However, the black mangabey L. aterrimus was an exception, as we detected the highest ER in P1, concomitant with the transect opening. The slight increase in ER observed with decreasing disturbance (from P2 to P4, Fig. 2), never approached the rates observed in P1, the passage with the highest disturbance. While our results seem to suggest that L. aterrimus required longer than any other species to re-occupy the areas where they had been disturbed, another more obvious explanation for this unexpected result may be the mangabey’s vocal behavior. L. aterrimus produces long-distance calls (up to 1 km in the allopatric grey-cheeked mangabey (Lophocebus albigena) (Brown 2020) used to signal (a) inter-group spacing and intra-group rallying, i.e., “whoop-gobble” and (b) alarm, i.e., karaou (Kingdon et al. 2013). As confirmed by the high proportion of heard vs. observed groups in P1 (76%), black mangabeys were more frequently heard calling during transect cutting (Supporting Table 1). Although the frequency of whoop-gobbles should not have been affected, we expect mangabeys to emit fewer alarm calls in passages with reduced disturbance (Campos and Fedigan 2014). This hypothesis could have been tested by investigating between-passages differences in call type frequency, which was not recorded in our study. Conversely, red colobuses and red-tailed monkeys were the least vocal (Supporting Table 1). Like in our study, Western red colobuses (Piliocolobus badius) in Taï National Park, Cote d’Ivoire, were found being relatively silent when spotting a potential predator (i.e., chimpanzee), taking advantage of Diana’ monkeys (Cercopithecus diana) as sentinels (Noë and Bshary, 1997). Similarly, in Taï, five primate species, including Western red colobuses (P. badius), Western black and white colobus (Colobus polykomos), Diana’s monkey (C. diana), the lesser white nosed monkey (Cercopithecus petaurista), the Campbell’s guenon (C. campbelli) and the sooty mangabey (Cercocebus atys), were found to emit fewer alarm calls when spotting a chimpanzee (thus avoiding signaling their presence), than when spotting a leopard (Zuberbühler et al. 1999). However, the opposite was observed in Kibale National Park, Uganda, where Ashy red colobuses (Piliocolobus tephrosceles) were extremely vocal when spotting a chimpanzee (Stanford, 1995). In SNP, P. tholloni were the most targeted species by poachers in the past (Thompson 2000). As a result, they might have responded to high hunting pressure from humans by reducing their alarm call rate, avoiding signaling their presence, like species such as the golden-bellied capuchin (Sapajus xanthosternos, Suscke et al. 2021) and the woolly monkey (Lagothrix poeppigii, Papworth et al. 2013).
Finally, we also observed a higher number of poly-specific groups with reduced disturbance (Table 1), consistent with other observational studies, where species associated to increase foraging and anti-predatory efficacy (Teelen, 2007), but split immediately when threatened (Stanford, 2002). In sum, the response to disturbance was species-specific, highlighting the need to consider site-specific behavioral and ecological features.
Group size (GS)
Our results showed that disturbance affected observed species’ group size (GS). As with the encounter rate, GS increased with time to transect cutting, with large group sizes being recorded in P4. However, a difference was still noticeable between P3 and P4, suggesting that a pause of 24 h to the initial cut were not sufficient to observe all individuals (Fig. 3). At the species level, this pattern was consistent in L. aterrimus, C. ascanius, and C. wolfi, all belonging to the sub-family Cercopithecinae. In contrast, the two Colobinae, P. tholloni and C. angolensis, showed no noticeable difference between passages, although the low number of C. angolensis observations in P4 (n = 2) did not allow any meaningful comparison.
These results can be explained by the ecological and behavioral differences between the Cercopithecinae and Colobinae sub-families. For example, red colobus monkeys are a highly social species, living in groups of up to 60 individuals (Maisels, et al., 1994), taking advantage of group cohesiveness as an antipredator strategy. In Kibale, Uganda, red colobus (P. tephrosceles) groups did not flee when noticing a predator, i.e., chimpanzees (Stanford 1995). Instead, females grabbed their juveniles and approached the adult males, standing their ground to the predator (Stanford 1995). As such, their naivety often reported when encountering humans (e.g., Nowak et al. 2016) could be the manifestation of this very anti-predatory behavior. Alternatively, the difference observed between sub-families could be triggered by anatomy: cheek pouches specific to Cercopithecinae are lacking in Colobinae. Cheek pouches allow to store and transport food for later ingestion somewhere safe in the canopy (Lambert 2005). As vigilance is higher the closer to a disturbance event (Campos and Fedigan 2014; Gaynor and Cords 2012), the observed increase in group size with time after transect cutting was likely due to guenons initially hiding in the canopy, with more individuals becoming visible with time to disturbance. Our study did not include behavioral observations, nor information about sex and age classes of the observed primates, which would have allowed investigating within-group differences in detection probability. For example, juvenile brown capuchins (Cebus apella) were reported feeding more often in suboptimal, risky locations, while showing lower vigilance levels (van Schaik and van Noordwijk 1989). In vervet monkeys (Chlorocebus pygerythrus), juveniles were the slowest age class in fleeing humans (Mikula et al. 2018). Therefore, in the guenons of our study, juveniles may have been the first to be observed after disturbance, while adult females and males were still hiding.
Average group size is estimated accurately only by independent studies involving direct follows of focal groups (Buckland et al., 2010a). However, primate group size is known to be driven by both predation risk (Croes et al. 2007; Stanford 1995) and food availability (Janson and Goldsmith 1995; Wrangham et al. 1993), which are geographically variable even within a single study area. For example, C. ascanius groups in Kibale, Uganda, were highly variable in number, ranging between 38 and 175 individuals (Table 3). As predation risk and food availability were likely to be geographically variable also in our study area (17,127 km2), we would have required following groups of different species in different areas in order to fully grasp variability in group size, which was impossible with the available resources. For this reason, we estimated group size during the survey. When comparing our observed and estimated group size (Table 2), we found that exact counts (i.e., observed group size) were consistently lower than estimates including acoustic detections, suggesting that rainforest habitat is particularly unsuitable in visually counting individual primates (Spaan et al., 2017). As it is difficult to accurately estimate the number of individuals from acoustic cues only, we suggest improving accuracy by combining visual and acoustic observations. Although our estimated group sizes were comparable to those from other sites obtained from focal groups follows (Table 3), we are aware that between–site comparisons are difficult. Depending on the composition of specific primate communities, ecological niches may or may not be occupied, affecting average group size. For this reason, in Table 3 we did not compare group sizes across allopatric species such as L. albigena, P. tephrosceles, and Colobus guereza, but only compared studies of the same species.
Density estimates (D)
Considering all species, our density estimates revealed the highest density in P4 and the lowest in P2 (Table 2). When using estimated group size, ER were similar between P3 and P4, but estimates were 12% higher for the second. This substantial difference must be ascribed to larger groups observed in P4 (Fig. 3), with more individuals being spotted the more time had passed after disturbance.
At the species level, sample size in P4 was too low for three species (P. tholloni, C. angolensis, and C. wolfi) not allowing to model the detection probability with distance. Consequently, we could not obtain a density estimate for these species in P4. Sample size was also low (< 30 sightings; Plumptre et al. 2013) for L. aterrimus (n = 17) and C. ascanius (n = 18), but sufficient to fit a detection function, which however, resulted in large confidence intervals (CI) of estimated densities. As a result, the CI obtained in P3 and P4 overlapped in both species, and the resulting estimates could not be considered reliable (Plumptre et al., 2013). However, the estimated mean density was similar between passages for L. aterrimus, and 17% higher in P4 for C. ascanius (Fig. 2), consistent with the pooled analysis of all species. These results suggest an interval of 10 days on average after transect cutting (i.e., the average interval between P1 and P4) being adequate for these species. We expect the same to be true for the other species in this study, showing comparable responses to disturbance (Fig. 2 and 3). Finally, the corrected density estimates used here, were similar to those obtained in other sites from follows of focal groups (Table 3) and supported by adequate sample size (Supporting Table 2), with the sole exception of the C. angolensis (n = 23). By that they support the efficiency of LTDS as an effective tool for estimating primate density, provided adherence to the method’s best practice guidelines (Buckland et al., 2010a).
Conclusions and practical recommendations
As expected, our study showed the negative effects of disturbance on both primate encounter rates and observed group sizes. It also showed that 24 h after disturbance were sufficient to detect most of the groups present along the transect, suggesting that primate groups should be counted not earlier than 24 h after the transect cutting. The same interval seemed adequate to assess group size of the two Colobinae, given their cohesiveness. However, due to increased vigilance after the transect cutting and / or avoidance of the area where the primates were disturbed, a longer time was required for the Cercopithecinae to resume their behavior under undisturbed conditions, allowing more accurate estimates of group size. For these species, we recommend the actual survey to take place a minimum of 7, ideally 10 days after cutting the transect, unless average group size has been quantified in a separate study involving direct follows of focal groups.
Despite efforts to minimize disturbance in our study, exact counts of group size were consistently lower than group size estimates. In situations where it is impossible or unfeasible to measure distances to individual primates, we recommend group size to be estimated combining visual and acoustic observations. To reduce bias, a team of ideally 3–5 trained observers (four in our study) should provide independent estimates of group size. As larger teams exert higher disturbance, it is important that the trade-off between number of independent estimates and disturbance is carefully weighted when designing the study. For example, a single observer could survey a transect almost unnoticed, maximizing the time spent in assessing group size, although this can be impractical for very large group sizes. Finally, estimates’ accuracy could be improved by using spatial hierarchical models, which estimate primate density by modelling detection probability and group size as a function of covariates (Cavada et al. 2017b).
By affecting both group encounter rate and group size, our study showed that observer disturbance is a critical factor to be considered in order to obtain reliable estimates of primate population density. Importantly, it also showed inter-specific differences in the way primates respond to disturbance, possibly resulting from different anti-predatory strategies as a response to hunting pressure. Some species responded by producing obvious alarm calls and subsequent hiding (e.g., L. aterrimus). Others, by remaining silent and gathering rather than fleeing (e.g., P. tholloni).
We wish to emphasize that our findings may apply differently to other areas of primate occurrence. The primates inhabiting the block South of SNP have been heavily poached in the past and still are hunted today. However, due to the remoteness and the size of the area, hunting pressure from humans was never continuous and did not affect the entire population simultaneously. In addition, only two surveys were conducted in the area before this study (Blake 2005; Grossmann et al. 2008). As a result, the groups encountered in our study, were very responsive to the presence of researchers, emitting frequent vocalizations (e.g., L. aterrimus) and avoiding the transect area for more than a week (e.g., all Cercopithecinae) after the first encounter with humans in P1. However, different primate communities are expected to show different behaviors according to habitat, niche occupation, and hunting pressure. Similarly, as thicker vegetation requires more cutting, the ideal interval between transect cutting and the survey will depend on the ground vegetation of the specific study area. It will also depend on the degree of habituation of the primates in study. For example, if the monkeys are used to encounter researchers (e.g., when pre-existing transects are used for monitoring purposes) their response might be reduced or not existent. It is therefore the responsibility of any future study to consider these factors when designing a primate survey using LTDS, in order to grant accurate assessments of primate density and abundance contributing to the implementation of effective conservation measures.
Data Availability Statement
The raw data used in this study are available via the Edmond Data Repository https://doi.org/10.17617/3.C0HBLR (Bessone et al. 2022).
References
Abernethy K, Maisels F, White LJ (2016) Environmental issues in central Africa. Annu Rev Environ Resour 41:1–33
Araldi A, Barelli C, Hodges K, Rovero F (2014) Density estimation of the endangered Udzungwa red colobus (Procolobus gordonorum) and other arboreal primates in the Udzungwa Mountains using systematic distance sampling. Int J Primatol 35(5):941–956
Bachand N, Arsenault J, Ravel A (2015) Urban household meat consumption patterns in Gabon, Central Africa, with a focus on bushmeat. Hum Dimens Wildl 20(2):147–158
Bessone M, Kühl HS, Hohmann G, Herbinger I, N’Goran KP, Asanzi P, Da Costa PB, Dérozier V, Fotsing DBE, Beka BI, Mpongo DI, Iyomi BI, Musubaho LKW, Fruth B (2020) Drawn out of the shadows: Surveying secretive forest species with camera-trap distance sampling. J Appl Ecol 57(5):963–974
Bessone M, Bondjengo N, Hausmann A, Herbinger I, Hohmann G, Kuehl H, Mbende M, N'Goran PK, Soliday V, Fruth B (2019) Inventaire de la biodiversité dans le bloc sud du parc national de la Salonga et développement d’une stratégie de suivi écologique pour améliorer la protection de la faune et de la flore menacées dans le parc. Final report. ICCN - WWF - MPI - LMU,
Bessone M, Kühl H, Herbinger I, N'Goran KP, Asanzi P, Da Costa BP, Dérozier V, Fotsing DBE, Ikembelo BB, Iyomi MD, Iyatshi IB, Kafando P, Kambere MA, Moundzoho DB, Musubaho KL, Fruth B (2022) Assessing the effects of survey-inherent disturbance on primate detectability: Recommendations for line transect distance sampling. Dataset. V1 edn. Edmond. https://doi.org/10.17617/3.C0HBLR
Blake S (2005). Monitoring the illegal killing of elephants- Central African forests: Final report on population surveys (2003–2004). MIKE- CITES- WCS, Washington DC, USA.
Bocian C (1997) Niche separation of black–and–white colobus monkeys (Colobus angolensis and C. guereza) in the Ituri Forest. PhD dissertation, City University of New York.
Brockelman WY, Tun AY, Pan S, Naing H, Htun S (2020) Comparison of point transect distance and traditional acoustic point-count sampling of hoolock gibbons in Htamanthi Wildlife Sanctuary. Myanmar Am J Primatol 82(12):e23198
Brown M (2020) Detecting an effect of group size on individual responses to neighboring groups in gray-cheeked mangabeys (Lophocebus albigena). Int J Primatol 41(2):287–304
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2001) Introduction to distance sampling estimating abundance of biological populations. Oxford University Press
Buckland ST, Marsden SJ, Green RE (2008) Estimating bird abundance: making methods work. Bird Conserv Int 18(S1):S91–S108
Buckland ST, Plumptre AJ, Thomas L, Rexstad EA (2010) Line transect sampling of primates: can animal-to-observer distance methods work? Int J Primatol 31(3):485–499
Campos FA, Fedigan LM (2014) Spatial ecology of perceived predation risk and vigilance behavior in white-faced capuchins. Behav Ecol 25(3):477–486
Carvalho J, Graham B, Bocksberger G, Maisels F, Williamson EA, Wich S, Sop T, Amarasekaran B, Bergl RA, Boesch C, Boesch H, Brncic T, Buys B, Chancellor R, Danquah E, Doumbé OR, Le Duc SY, Galat-Luong A, Ganas J, Gatti S et al (2021) Predicting range shifts of African apes under global change scenarios. Diver Distrib 27(9):1663–1679
Cavada N, Ciolli M, Barelli C, Rovero F (2017a) Optimizing field and analytical procedures for estimating densities of arboreal and threatened primates in tropical rainforest. Am J Primatol 79(9):e22666
Cavada N, Ciolli M, Rocchini D, Barelli C, Marshall AR, Rovero F (2017b) Integrating field and satellite data for spatially explicit inference on the density of threatened arboreal primates. Ecol Appl 27(1):235–243
Cavada N, Tenan S, Barelli C, Rovero F (2019) Effects of anthropogenic disturbance on primate density at the landscape scale. Conserv Biol 33(4):873–882
Chapman CA, Lambert JE (2000) Habitat alteration and the conservation of African primates: case study of Kibale National Park. Uganda Am J Primatol 50(3):169–185
Chapman CA, Struhsaker TT, Skorupa JP, Snaith TV, Rothman JM (2010) Understanding long-term primate community dynamics: implications of forest change. Ecol Appl 20(1):179–191
Chapman CA, Bicca-Marques JC, Dunham AE, Fan P, Fashing PJ, Gogarten JF, Guo S, Huffman MA, Kalbitzer U, Li B (2020) Primates Can Be a Rallying Symbol to Promote Tropical Forest Restoration. Folia Primatol:1–19.
Croes BM, Laurance WF, Lahm SA, Tchignoumba L, Alonso A, Lee ME, Campbell P, Buij R (2007) The influence of hunting on antipredator behavior in Central African monkeys and duikers. Biotropica 39(2):257–263
Dacier A, de Luna AG, Fernandez-Duque E, Di Fiore A (2011) Estimating population density of Amazonian titi monkeys (Callicebus discolor) via playback point counts. Biotropica:135–140.
de Luna AG, Link A (2018) Distribution, population density and conservation of the critically endangered brown spider monkey (Ateles hybridus) and other primates of the inter-Andean forests of Colombia. Biodivers Conserv 27(13):3469–3511
Defler TR, Pintor D (1985) Censusing primates by transect in a forest of known primate density. Int J Primatol 6(3):243–259
Fa JE, Olivero J, Farfán MA, Lewis J, Yasuoka H, Noss A, Hattori S, Hirai M, Kamgaing TO, Carpaneto G (2016) Differences between pygmy and non-pygmy hunting in Congo Basin forests. PLoS ONE 11(9):e0161703
Ferrari SF, Chagas RR, Souza-Alves JP (2010) Line transect surveying of arboreal monkeys: problems of group size and spread in a highly fragmented landscape. Am J Primatol 72(12):1100–1107
Galat–Luong A (1975) Notes preliminaires sur l’écologie de Cercopithecus ascanius schmidti dans les environs de Bangui (RCA). La Terre et la vie.
Gautier–Hion A (2013) in Butynski TM, Kingdon J, Kalina J (Eds.) Mammals of Africa. Vol.II, Primates, Bloomsbury Publishing, London.
Gaynor KM, Cords M (2012) Antipredator and social monitoring functions of vigilance behaviour in blue monkeys. Anim Behav 84(3):531–537
Gestich CC, Caselli CB, Nagy-Reis MB, Setz EZ, da Cunha RG (2017) Estimating primate population densities: The systematic use of playbacks along transects in population surveys. Am J Primatol 79(2):e22586
Grossmann F, Hart JA, Vosper A, Ilambu O (2008) Range occupation and population estimates of bonobos in the Salonga National Park: application to large-scale surveys of bonobos in the Democratic Republic of Congo. In: Furuichi T, Thompson J (eds) The Bonobos: Behavior. Springer, Ecology and Conservation, pp 189–216
Hansen MF, Nawangsari VA, van Beest FM, Schmidt NM, Fuentes A, Traeholt C, Stelvig M, Dabelsteen T (2019) Estimating densities and spatial distribution of a commensal primate species, the long-tailed macaque (Macaca fascicularis). Conserv Sci Pract 1(9):e88
Hassel-Finnegan HM, Borries C, Larney E, Umponjan M, Koenig A (2008) How reliable are density estimates for diurnal primates? Int J Primatol 29(5):1175–1187
Hofner AN, Jost Robinson C, Hall ES, Capel T, Astaras C, Linder JM (2020) Surveying primates in northeastern Korup National Park. Cameroon A Longitudinal Comparison Afr Primates 14:35–44
Horn AD (1987) The socioecology of the black mangabey (Cercocebus aterrimus) near Lake Tumba. Zaire Am J Primatol 12(2):165–180
Hurtado CM, Serrano-Villavicencio J, Pacheco V (2016) Population density and primate conservation in the Noroeste Biosphere Reserve, Tumbes. Peru Revista Peruana De Biología 23(2):151–158
IUCN (2021) The IUCN Red List of Threatened Species. Version 2021–1 <https://www.iucnredlist.org>
Janson CH, Goldsmith ML (1995) Predicting group size in primates: foraging costs and predation risks. Behav Ecol 6(3):326–336
Junker J, Blake S, Boesch C, Campbell G, Toit Ld, Duvall C, Ekobo A, Etoga G, Galat-Luong A, Gamys J et al (2012) Recent decline in suitable environmental conditions for African great apes. Divers Distrib 18(11):1077–1091
Kalan AK, Mundry R, Wagner OJ, Heinicke S, Boesch C, Kühl HS (2015) Towards the automated detection and occupancy estimation of primates using passive acoustic monitoring. Ecol Indic 54:217–226
Kingdon J, Happold D, Butynski T, Hoffmann M, Happold M, Kalina J (2013) Mammals of Africa, Volume 1. A&C Black.
Kühl HS, Sop T, Williamson EA, Mundry R, Brugière D, Campbell G, Cohen H, Danquah E, Ginn L, Herbinger I et al (2017) The Critically Endangered western chimpanzee declines by 80%. Am J Primatol 79(9):e22681
Kühl HS, Maisels F, Ancrenaz M, Williamson EA (2008) Best practice guidelines for the surveys and monitoring of great ape populations. Gland, Switzerland: IUCN SSC Primate Specialist Group (PSG). 32 pp.
Kümpel NF, Milner-Gulland E, Rowcliffe JM, Cowlishaw G (2008) Impact of gun-hunting on diurnal primates in continental Equatorial Guinea. Int J Primatol 29(4):1065–1082
Lambert JE (2005) Competition, predation, and the evolutionary significance of the cercopithecine cheek pouch: the case of Cercopithecus and Lophocebus. American J Phys Anthropol 126(2):183–192
Linder JM, Oates JF (2011) Differential impact of bushmeat hunting on monkey species and implications for primate conservation in Korup National Park. Cameroon Biol Conserv 144(2):738–745
Maisels F, Gautier-Hion A, Gautier J-P (1994) Diets of two sympatric colobines in Zaire: more evidence on seed-eating in forests on poor soils. Int J Primatol 15(5):681–701
Marshall AR, Lovett JC, White PC (2008) Selection of line-transect methods for estimating the density of group-living animals: lessons from the primates. Am J Primatol 70(5):452–462
McGraw S (1994) Census, habitat preference, and polyspecific associations of six monkeys in the Lomako Forest. Zaire Am J Primatol 34(4):295–307
McLester E, Brown M, Stewart FA, Piel AK (2019) Food abundance and weather influence habitat-specific ranging patterns in forest-and savanna mosaic-dwelling red-tailed monkeys (Cercopithecus ascanius). Am J Phys Anthropol 170(2):217–231
Mikula P, Šaffa G, Nelson E, Tryjanowski P (2018) Risk perception of vervet monkeys Chlorocebus pygerythrus to humans in urban and rural environments. Behav Processes 147:21–27
Mitani JC, Sanders WJ, Lwanga JS, Windfelder TL (2001) Predatory behavior of crowned hawk-eagles (Stephanoaetus coronatus) in Kibale National Park. Uganda Behav Ecol Sociobiol 49(2):187–195
Moore J, Pine W, Mulindahabi F, Niyigaba P, Gatorano G, Masozera M, Beaudrot L (2020) Comparison of species richness and detection between line transects, ground camera traps, and arboreal camera traps. Anim Conserv 23(5):561–572
N'Goran PK, Kuehl H, Hohmann G, Fruth B, Herbinger I, Mbende M (2016) Biomonitoring dans le Bloc Sus du Parc national de la Salonga, RDC. Protocole general de collecte de donnees sur les mammiferes. ICCN - WWF - MPI - LMU,
Nichols JD, Williams BK (2006) Monitoring for conservation. Trends Ecol Evol 21(12):668–673
Noë R, Bshary R (1997) The formation of red colobus-diana monkey associations under predation pressure from chimpanzees. Proc R Soc Lond B264:253–259
Nowak K, Hill RA, Wimberger K, Al R (2016) Risk-taking in Samango monkeys in relation to humans at two sites in South Africa. In: Dore KM, Riley EP, Fuentes A (eds) Ethnoprimatology. Springer, pp 301–314
Paddock CL, Bruford MW, McCabe GM (2020) Estimating the population size of the Sanje mangabey (Cercocebus sanjei) using acoustic distance sampling. Am J Primatol 82(2):e23083
Papworth S, Milner-Gulland E, Slocombe K (2013) Hunted woolly monkeys (Lagothrix poeppigii) show threat-sensitive responses to human presence. PLoS ONE 8(4):e62000
Peres CA (1999) General guidelines for standardizing line-transect surveys of tropical forest primates. Neotropical Primates 7(1):11–16
Plumptre AJ, Cox D (2006) Counting primates for conservation: primate surveys in Uganda. Primates 47(1):65–73
Plumptre AJ, Nixon S, Kujirakwinja DK, Vieilledent G, Critchlow R, Williamson EA, Kirkby AE, Hall JS (2016) Catastrophic decline of world’s largest primate: 80% loss of Grauer’s Gorilla (Gorilla beringei graueri) population justifies critically endangered status. PLoS ONE 11(10):e0162697
Plumptre AJ, Reynolds V (1994) The effect of selective logging on the primate populations in the Budongo Forest Reserve, Uganda. J Appl Ecol: 631–641.
Plumptre AJ, Sterling EJ, Buckland ST (2013) Primate census and survey techniques. Primate ecology and conservation: a handbook of techniques Oxford University Press, Oxford:10–26.
Quinten MC, Nopiansyah F, Hodges JK (2016) First estimates of primate density and abundance in Siberut National Park, Mentawai Islands. Indonesia Oryx 50(2):364–367
R Core Team (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Version 4.0.5. Available from http://www.r-project.org/.
Remis MJ, Jost Robinson CA (2012) Reductions in primate abundance and diversity in a multiuse protected area: Synergistic impacts of hunting and logging in a Congo Basin forest. Am J Primatol 74(7):602–612
Rosenbaum B, O’Brien TG, Kinnaird M, Supriatna J (1998) Population densities of Sulawesi crested black macaques (Macaca nigra) on Bacan and Sulawesi, Indonesia: effects of habitat disturbance and hunting. Am J Primatol 44(2):89–106
Rovero F, Struhsaker TT, Marshall AR, Rinne TA, Pedersen UB, Butynski TM, Ehardt CL, Mtui AS (2006) Abundance of diurnal primates in Mwanihana forest, Udzungwa Mountains. Tanzania Int J Primatol 27(3):675–697
Semel BP, Karpanty SM, Vololonirina FF, Rakotonanahary AN (2020) Eyes in the sky: Assessing the feasibility of low-cost, ready-to-use Unmanned Aerial Vehicles to monitor primate populations directly. Folia Primatol 91(1):69–82
Shanee S, Allgas N, Ocampo-Carvajal C, Shanee N (2021) A high-diversity primate community in a mid-elevation flooded forest, the Jungla de Los Monos Community Reserve. Peru Primates 62(1):189–197
Sheppard DJ (2000) Ecology of the Budongo Forest redtail: Patterns of habitat use and population density in primary and regenerating forest sites. PhD dissertation, University of Calgary.
Skorupa JP (1987) Do line-transect surveys systematically underestimate primate densities in logged forests? Am J Primatol 13(1):1–9
Spaan D, Ramos-Fernández G, Schaffner CM, Pinacho-Guendulain B, Aureli F (2017) How survey design affects monkey counts: a case study on individually recognized spider monkeys (Ateles geoffroyi). Folia Primatol 88(5):409–420
Spaan D, Burke C, McAree O, Aureli F, Rangel-Rivera CE, Hutschenreiter A, Longmore SN, McWhirter PR, Wich SA (2019) Thermal infrared imaging from drones offers a major advance for spider monkey surveys. Drones 3(2):34
Spaan D, Ramos-Fernández G, Bonilla-Moheno M, Schaffner CM, Morales-Mávil JE, Slater K, Aureli F (2020) Anthropogenic habitat disturbance and food availability affect the abundance of an endangered primate: a regional approach. Mamm Biol 100(3):325–333
Stan Development Team (2021) RStan: the R interface to Stan. R Package Version 2(21):2
Stanford CB (1995) The influence of chimpanzee predation on group size and anti-predator behaviour in red colobus monkeys. Anim Behav 49(3):577–587
Struhsaker T (1975) The Red Colobus Monkey. University Chicago Press, Chicago
Struhsaker T (1978) Food habits of five monkey species in the Kibale Forest, Uganda. Recent Advances in Primatology 1:225–248
Struhsaker T (1980) Comparison of the behaviour and ecology of red colobus and redtail monkeys in the Kibale Forest. Uganda Afr J Ecol 18(1):33–51
Struhsaker T (1997) Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. University Press of Florida.
Suscke P, Presotto A, Izar P (2021) The role of hunting on Sapajus xanthosternos’ landscape of fear in the Atlantic Forest. Brazil Am J Primatol 83(5):e23243
Teelen S (2007) Influence of chimpanzee predation on associations between red colobus and red-tailed monkeys at Ngogo, Kibale National Park. Uganda Int J Primatol 28(3):593–606
Thomas SC (1991) Population densities and patterns of habitat use among anthropoid primates of the Ituri Forest, Zaire. Biotropica:68–83.
Thompson JAM (2000) Conservation of Thollon’s red colobus Piliocolobus tholloni, Democratic Republic of Congo. Afr Primates 4:27–32
Tutin CE, Fernandez M (1984) Nationwide census of gorilla (Gorilla g. gorilla) and chimpanzee (Pan t. troglodytes) populations in Gabon. Am J Primatol, 6(4), 313–336.
Tyukavina A, Hansen MC, Potapov P, Parker D, Okpa C, Stehman SV, Kommareddy I, Turubanova S (2018) Congo Basin forest loss dominated by increasing smallholder clearing. Sci Advances 4 (11):eaat2993.
van Schaik CP, van Noordwijk MA (1989) The special role of male Cebus monkeys in predation avoidance and its effect on group composition. Behav Ecol Sociobiol 24(5):265–276
van Vliet N, Nebesse C, Gambalemoke S, Akaibe D, Nasi R (2012) The bushmeat market in Kisangani, Democratic Republic of Congo: implications for conservation and food security. Oryx 46(2):196–203
Windfelder TL, Lwanga JS (2004) Group fission in red–tailed monkeys (Cercopithecus ascanius) in Kibale National Park, Uganda. In Gleen ME, Cords, M: The guenons: Diversity and adaptation in African monkeys. Springer, pp 147–159.
Wrangham RW, Gittleman JL, Chapman C (1993) Constraints on group size in primates and carnivores: population density and day-range as assays of exploitation competition. Behav Ecol Sociobiol 32(3):199–209
Yanuar A, Supriatna J (2018) The status of primates in the southern Mentawai Islands. Indonesia Primate Conserv 32(1):193–203
Zuberbühler K, Jenny D, Bshary R (1999) The predator deterrence function of primate alarm calls. Ethology 105(6):477–490
Acknowledgements
This work was funded by Liverpool John Moores University and by the Kreditanstalt für Wiederaufbau (KfW Group) on behalf of the German Government and WWF Germany. We are grateful to all people that assisted data collection: M. Bofeko, R. Booto, B. Katembo, J. Kukumanga, S. Mwanduku, P. Musenzi, R. Ratsina, P. Bondo, G. Mbambe, W. Bokungu, P. Bokembi, E. Way, P. Ingange, J. Iyondo, B. Nkoy, A. Bomondjo. We thank S. Abedi, J. Eriksson, M. Etike, A. Kabamba, G. Kasiala, R. Keller, L. Lokumu, C. Longanga, E. Lopongo, A. Louat, S. Matungila, M. Mbende, O. Nelson, for facilitating fieldwork; S. Mbongo, G. Kayembe, C. Kabamba, L. Nsenga, M. and T. de Sousa, for logistic support. For administrative support, we thank (in alphabetical order) M. Crofoot, K. George, B. Grothe, R. Leatherbarrow, R. McElreath, Z. Pereboom, A. Tattersall, M. Tomasello, P. Wheeler, W. Wickler and M. Wikelski. In DRC, we thank the Institut Congolais pour la Conservation de la Nature, the Ministère de la Recherche Scientifique and the Unité de Gestion du Parc National de la Salonga, for permitting and supporting research in Salonga National Park.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bessone, M., Kühl, H.S., Hohmann, G. et al. Assessing the effects of survey-inherent disturbance on primate detectability: Recommendations for line transect distance sampling. Primates 64, 107–121 (2023). https://doi.org/10.1007/s10329-022-01039-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-022-01039-4