Abstract
The rising global population is inducing a fast increase in the amount of municipal waste and, in turn, issues of rising cost and environmental pollution. Therefore, alternative treatments such as waste-to-energy should be developed in the context of the circular economy. Here, we review the conversion of municipal solid waste into energy using thermochemical methods such as gasification, combustion, pyrolysis and torrefaction. Energy yield depends on operating conditions and feedstock composition. For instance, torrefaction of municipal waste at 200 °C generates a heating value of 33.01 MJ/kg, while the co-pyrolysis of cereals and peanut waste yields a heating value of 31.44 MJ/kg at 540 °C. Gasification at 800 °C shows higher carbon conversion for plastics, of 94.48%, than for waste wood and grass pellets, of 70–75%. Integrating two or more thermochemical treatments is actually gaining high momentum due to higher energy yield. We also review reforming catalysts to enhance dihydrogen production, such as nickel on support materials such as CaTiO3, SrTiO3, BaTiO3, Al2O3, TiO3, MgO, ZrO2. Techno-economic analysis, sensitivity analysis and life cycle assessment are discussed.
Similar content being viewed by others
Introduction
Population growth at a greater pace, rapid urbanization, higher living standards and sophisticated lifestyle have a very large impact on consumption pattern and the generation of wastes. Industrial and domestic activities generate waste which may be degradable or non-degradable, hazardous or non-hazardous to the surrounding environment and cause adverse health effects on the living beings. Waste materials can be either in solid or in liquid forms and fall under different categories such as municipal solid wastes, industrial wastes, medical wastes, radioactive wastes, chemical wastes and agricultural waste.
The management of wastes is very important to reduce the adverse effect on the environment. Statistics says that the annual growth rate of municipal solid waste ranges from 3.2 to 4.5% in developed countries and 2–3% in developing countries (Tang et al. 2018; Monisha et al. 2021).
The emanation of waste in solid forms is of very large proportion and highly complex to handle. The municipal solid waste generation report from the United Nations affirms that almost 100% of the goods procured by consumers get converted to waste in a span of 6 months (Al Rayaan 2021; Nanda et al. 2021; Gunarathne et al. 2019).
Though the recyclable waste proportion is estimated closer to 80%, about 90 to 95% of the wastes reaches the landfills due to improper handling and the disposal of them (Gaeta-bernardi and Parente 2016). All developing countries are facing challenges in collecting and disposing the waste. In many countries, food wastes and plastics are the major contributors of municipal solid wastes (Zhou et al. 2015; Khan et al. 2016). Furthermore, due to the outbreak of coronavirus disease followed by sudden lockdown across the world, the drastic increase in the usage of plastic materials, i.e., face mask and gloves, has increased the municipal solid waste generation rate with approximately more than 6000 tons per day in Southeast Asian countries (Yang et al. 2021; Cai et al. 2021).
A few decades ago, waste-to-energy technologies were introduced to produce energy and valuable products from waste and paved way for finding technologies in processing wastes based on a tool called ‘waste hierarchy’—reduce, reuse, recycle and recover to landfill (Voss et al. 2021; Smidt et al. 2010). The aim of waste hierarchy is to reduce the waste generation and to increase the recycle and reuse of the waste materials. Waste hierarchy method helps in reducing greenhouse gas and pollutants which harm the environment (Sánchez et al. 2015; Sivaprakash et al. 2011; Rajamohan et al. 2010).
The paper and plastic are the major fraction in municipal solid waste ranging from 50 to 80% while the remaining fraction is contributed by textile, wood, sewage waste, kitchen wastes and laundry waste. The composition of municipal solid waste may vary depending on local economy, climatic condition, lifestyle and infrastructure. The waste generation of an area is proportional to the average income of the people. Demolition and construction activities consist of used wood and may contain 2–3% of non-wood such as glass, plastics, concrete and scrap metals.
The annual quantity of solid wastes generated in India has increased from 6 million tons in 1947 to 48 million tons in 1997 and to 90 million tons in 2009 and also expected to increase by 2047 up to 300 million tons (Gupta et al. 2015). The global generation rate of municipal solid waste is estimated as 1.3 billion tons per year in 2012 and expected to rise 2.2 billion tons annually by 2025 (Couto et al. 2016). The management of municipal solid waste is commonly reduced by incineration, about mass reduction (70–80%) and volume reduction (80–90%) and utilized as electricity with net electrical efficiency of 30% (Chan et al. 2019).
The treatment of municipal solid waste includes dumping in landfills, incineration of waste and waste-to-energy treatments such as biological process, chemical process, bio-chemical conversion and thermochemical processes (Fig. 1).
Plastic materials possess certain intrinsic properties similar to fuels which makes them as a throughput material for the production of heat, steam and several other fuels through thermochemical methods. In addition, biomass from different sources can be used as renewable sources for energy production. During thermochemical treatments when compared to fossil fuels, biomass emits a reduced amount of SOx, NOx and greenhouse gases (Acharya et al. 2015; Rawoof et al. 2021; Rajamohan et al. 2008). The waste-to-energy is one of the most promising alternative methods in waste management strategies.
About 80% of energy is derived from the petroleum products to satisfy the energy demand around the world. The thermochemical treatment of municipal waste is gaining momentum as the most promising waste-to-energy production method mainly due to sustainability. The studies showed that less than 100,000 m2 of land is enough to treat 1 metric ton per year of waste for 30 years, while 3,000,000 m2 is necessary for landfilling of 30 metric tons of municipal solid waste (Arena 2012). The concentration of organic and inorganic contaminant can be securely disposed and utilized by thermochemical treatment without any threats.
The by-products obtained from thermochemical method such as bottom ash, solid residues and metal or non-metal slags are recyclable and can be reused. The emission of greenhouse gas and other pollutants are significantly minimized and estimated that about one ton of CO2 is retrieved when compared to landfilling. Life cycle assessment studies considered that waste-to-energy technology has a less impact on environment and can be used as a source for power generation. The electricity and process heat from thermochemical treatment method can be utilized for both industrial facilities and for residential or commercial power requirements (Arena 2012; Saidi et al. 2020).
This review focuses on (i) the outlook of various thermochemical treatment methods of municipal solid waste viz., organic, paper, plastic and mixed municipal solid waste, (ii) latest reforming methods and catalysts available for enhanced fuel production and (iii) practical challenges involved in implementation on real-time applications.
Thermochemical conversion technologies
Biomass is a renewable, clean and green source to produce fuels that can cater the energy needs. However, the direct usage of biomass as biofuels has many limitations such as poor calorific value, undesirable moisture content, abnormal composition and properties. Thermochemical techniques offer pathways that alleviate these disadvantages and drastically reduce the undesired by-products by optimizing the operating conditions (Ong et al. 2020).
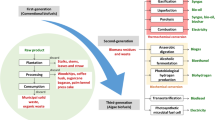
Combustion, gasification, pyrolysis, hydrothermal carbonization and torrefaction are the thermochemical methods that aid in the production of biofuels from lignocellulosic and non-lignocellulosic materials. Figure 2 shows an outlay on various thermochemical treatments of municipal solid waste for biofuel production and reforming processes.
Torrefaction into solid products
Biomass can be converted into char, coke and biochar which have similar properties to that of coal. Biochar derived from torrefaction possess properties of coal fuel. Torrefaction is the mild pyrolysis process carried out in the absence of oxygen or in an inert atmosphere at 200–300 °C to produce biofuel with improved fuel characteristics; the torrefied solid biomass has high calorific value, low moisture content and other properties such as grindability and hydrophobicity (Tran et al. 2013). The aim of torrefaction is to produce solid fuel as an alternative to coal and to produce materials with high hydrophobicity (Chen et al. 2021).
The toasted biomass contains much of volatile matter and requires high residence time to reduce the volatile matter. Combustion reactions are suppressed because of low oxygen supply. Improved grindability reduces power cost required for size reduction and enables the displacement of coal usage. Additionally, the lower equilibrium moisture content of the torrefied biomass has the advantages of storage ease and higher heating value (Agar et al. 2012).
Gaseous products produced in torrefaction process are classified into permanent gas (CO and CO2) and condensable gas (acetic acid and water). Torrefaction is also reported as a thermal pre-treatment process of biochar production to improve the energy density and used as a low-moisture feedstock to produce energy. Torrefaction may be light, mild or severe based on the temperature input required to consume the lignocellulosic contents of the biomass. In torrefaction process products can be in all three forms and most of those can be used as fuels. Some of the products received in torrefaction are H2, CO2, toluene and benzene (gaseous form); H2O, acetic acids, alcohols, aldehydes and ketones (liquid form); and char and ash (solid form) (Mamvura et al. 2020).
The advantages of torrefaction process include higher heating value, higher hydrophobicity, grindability, lower O/C and H/C ratios and low moisture content of the coal produced (Chen et al. 2015). Hydrothermal carbonization was considered as the most used thermal technology where the yield of solid biofuel was around 38% when compared to pyrolysis, torrefaction and gasification (15%, 15% and 3%). The hydrothermal carbonized biofuel showed more advantages than fossil and also showed certain constraints with heating value, bulk density, energy density and grindability (Angulo-Mosquera et al. 2021).
Pyrolysis to produce liquids
Biomass can be converted into liquid fuels, namely, bio-oil, bio-diesel and bio-ethanol by using pyrolysis, transesterification and fermentation, respectively. The scope of this review is limited to production of bio-oil through pyrolysis as the other methods are non-thermochemical techniques. In pyrolysis technology waste materials are degraded thermally with restricted supply of air or oxygen at 300–600 °C to generate the energy in the form of fuel, biochar, syngas and biofuel precursors. Pyrolysis is an irreversible process; the materials which undergo the process are continuously subjected to changes in physical and chemical composition (Mamvura et al. 2020).
Pyrolysis process has a wide range of application in chemical industries for the production of methanol, biochar and activated charcoal with the only limitation being emission of harmful gases which causes adverse effect in the environment (Hasan et al. 2021). The biochar produced through pyrolysis assists in adsorption and used in heavy metal remediation, soil amelioration and carbon sequestration. The biochar is also used as a soil conditioner which can alleviate about 0.75 gigatons of carbon or 2.75 gigatons of carbon dioxide per year if used globally on an average till 2050 (Shahbaz et al. 2021).
The fuel product of pyrolysis, called as pyrolytic oil (dark brown color liquid) is characterized by large water and oxygen content, high acidity with pH from 2 to 4, low vapor pressure, high viscosity, weak stability and low heating value (16–19 MJ/Kg) (Kan et al. 2020). The bonds in hydrocarbons are thermally broken to produce bio-oil at the temperature range of 400–800 °C. Pyrolysis can be slow, fast, catalytic or microwave-assisted. The main products in slow and fast pyrolysis process are biochar and bio-oil, respectively, where slow pyrolysis incurs low heating with long residence time and fast pyrolysis incurs high heating with short residence time (Chen et al. 2021).
Bio-oil contains more than 300 organic compounds; however, bio-oil is not directly used as a fuel due to several disadvantages. The by-products generated through the bio-oil production depend on temperature, particle size, heating time, catalyst used and heating rate. High-quality bio-oil can be produced by using noble metal-supported catalyst and further processing in refineries (Ong et al. 2020). Biofuel production from different feedstocks with the optimum temperature condition reported by several authors is presented in Table 1.
Gasification and combustion–gaseous product
Gasification is a process in which biomass is burnt in limited supply of air or oxygen to generate producer gas. Producer gas is composed of H2, CO, CH4 and CO2 with certain by-products including H2S, COS, CS2, NH3 and HCN (depends on feed). The temperature range of gasification is generally between 600 and 1200 °C (Chen et al. 2021). The producer gas can used as a direct fuel or can be converted into profitable products like H2, synthetic natural gas (methanation) and diesel, jet fuel, gasoline (Fischer–Tropsch diesel process), methanol, ethanol and dimethyl ether (Shahbaz et al. 2021).
The product consists of mixture of gases depending on various parameters such as gasifying agent, type of gasifiers used, equivalence ratio, temperature, feedstock particle size, moisture content and catalyst. The gasification process is further classified into steam gasification, supercritical water gasification and catalytic gasification depending on the gasifying agent. The main drawback of using syngas for downstream application is high concentration of impurities such as tar, particulates, alkali chloride and sulfur species. Various hot syngas clean-up systems have been developed for the removal of such impurities.
In combustion technology, heat is the main product and yield depends upon feedstock, gasifier and temperature (750–1500 °C). Almost all solid wastes can be treated using combustion. Other than electricity production, combustion is also used for drying, pre-heating and steam generation in industries. The carbon-based materials like coal are majorly used for production of energy, releasing large amount of CO2 to the environment (Chan et al. 2019).
According to the International Energy Agency, coal contributes to approximately 44% of CO2 emission in the range of 0.34–0.39 kgCO2/kWh. The utilization of biomass along with coal can be a potential approach for the overall CO2 reduction (Shahbaz et al. 2021). Many authors reported biofuel production from different feedstocks and those are presented in Table 2.
Thermochemical treatment of municipal solid waste
The disadvantages caused by generation of enormous amount of municipal solid waste include environmental, economic and social offsets. These wastes, if not collected or processed using appropriate treatment or management strategies lead to spread of diseases through insects, microbial contamination, air, water and land pollution. However, the organic content of the municipal solid waste can be exploited to produce energy thereby displacing the usage of fossil fuels. The thermochemical treatment methods can be applied for the processing of municipal solid wastes for production of variety of energy-oriented products.
Thermochemical treatment of organic waste
Municipal solid waste contains significant amount of organic waste such as kitchen or food waste, yard waste and sludge waste. All the foresaid waste composition may differ based on the region and may contribute nearly one-third of the municipal solid waste.
Catalytic co-pyrolysis of kitchen waste and tire waste under N2 atmosphere was investigated and the characteristics were analyzed through thermogravimetric analysis–Fourier transform infrared spectroscopy (TGA–FTIR) and pyrosis–gas chromatography/mass spectroscopy (Py–GC/MS). TG-FTIR result showed that the released producer gas has a composition of CO2, CO, NO, NH3, SO2, C–H and C=C groups. The kinetic studies showed that the activation energy declined at 5:5 feedstock ratio and improved thermal degradation was also noted at same ratio (5:5) (Chen et al. 2019).
A pyrolysis process carried out using waste cereals and peanut crisps up to 800 °C and the samples were analyzed using thermogravimetric analyzer at discontinuous temperature range of 480–530, 550–600, 650–700 and 750–800 °C. The highest composition of hydrogen was observed approximately at 750–800 °C. The gas evolution from waste cereal was increased at approximately 540 °C. The minimum heating value of waste cereal and waste peanut crisps was reported to be 11.2 MJ/m3 and 17.6 MJ/m3, respectively. The yield of biochar was 22% with heating value of 31.44 MJ/kg. Traces of heavy metals were also present in biochar (Grycová et al. 2016).
Air gasification of wood pellets, wood chips and grass pellets were investigated in an electrically heated bubble fluidized bed at temperature of 650, 750 and 800 °C with equivalence ratio 0.08, 0.13 and 0.16, respectively. The experiment inferred that at 800 °C and 0.16 equivalence ratio the pressure drop remained constant and was preferable to oxidize the wood chips. The corresponding hydrogen and carbon monoxide content were 16.9% and 20% for wood chips and for wood pellets 17.2% and 18.8%. Gasification of grass pellets was not successful due to agglomeration and reduced carbon conversion. The carbon conversion of wood chips and pellets was 75% and 70%, respectively (Bandara et al. 2021).
The production of biofuel has also been done through dry torrefaction as a pre-treatment of yard wastes. Experiment was conducted at 170, 200, 250 and 300 °C temperature in a tubular reactor under N2, CO2 and flue gas (25:75 of CO2:N2 vol%) atmosphere. Temperature played a vital role than carrier gas, increase in temperature increased the higher heating value and decreased the mass and energy yield. Among the three gases, FTIR showed that CO2 is considered as the best gas with an elevated higher heating value and energy yield. N2 gas also provided appreciable higher heating value at high temperature. Flue gas proved to be the least effective carrier gas even at 250 °C. Proximate analysis showed that torrefaction reduced moisture content and increased hydrophobicity (Jaideep et al. 2021).
The torrefaction of lignocellulosic biomass was investigated along with the analysis of chemical and structural changes during thermal treatment. The sample was torrefied at 330 ºC that increased the acid insoluble residues from approximately 30–38% due to formation of condensed aromatic compounds. The non-protonated aromatic carbon fraction was observed about 60% of total aromatic carbon at 330 ºC which shows the large aromatic clusters. Solid-state nuclear magnetic resonance indicated the increased aromaticity about 36–60% and changes in methoxyl functional groups. The quantitative structural analysis of the sample revealed the main changes in chemical composition of biomass during torrefaction (Park et al. 2013).
Recently, an attempt was made by integrating anaerobic digestion and thermochemical treatments, namely, gasification, pyrolysis and hydrothermal carbonization on solid waste treatment. This article evaluated six types of coupling, viz., anaerobic digestion–gasification, gasification–anaerobic digestion, pyrolysis–anaerobic digestion, anaerobic digestion–pyrolysis, anaerobic digestion–hydrothermal carbonization and hydrothermal carbonization–anaerobic digestion. Many benefits including improved degradation rates of waste, enhanced process efficiency, recycling and reutilization were observed. Particularly, the pyrolytic product (biochar) showed improved ability and versatility for various applications (Sikarwar et al. 2021).
Thermochemical treatment of paper waste
Paper waste sludge, a major waste residue after papermaking can develop into a large source of pollution without effective disposal. Hence, appropriate management and disposal methods are required.
Combustible solid waste and paper mill sludge were treated using co-pyrolysis method and the degradation characteristics were investigated using FTIR and Py–GC/MS. The proportion of paper mill sludge used in the combustible solid waste-paper mill sludge blends was 10%, 30% and 50%. Out of the 11 pyrolysis products identified, acidic products were found to be more. The percentage of alcohol content was maximum for 10% paper mill sludge blend. Increase in paper mill sludge ratio increased the residue mass from 17.74% to 30.47% and slightly increased with the temperature. The product distribution was highly influenced by the synergistic interaction between combustible solid waste and paper mill sludge based on the blend ratio (Fang et al. 2017b).
The temperature, characteristic index, interaction and activation energy on co-pyrolysis of municipal solid waste (M) and paper mill sludge (P) and blends of municipal solid waste and paper mill sludge such as 90M10P, 70M30P, 50M50P, 30M70P, 10M90P were studied. MgO and activated carbon were used as additives. The experiment inferred that the pyrolysis temperature increased with paper mill sludge proportion and decreased with the charge of additives. The result showed that 90M10P and 70M30P can be opted as preferable ratios for pyrolysis process. The average activation energy achieved in 5% MgO and 5% activated carbon additives were 237.42 kJ/mol and 239.44 kJ/mol, respectively, and hence can be considered as potential additives. The synergistic interaction between the wastes at high temperature revealed that 90M10P as a perfect blend ratio (Fang et al. 2017a).
The pyrolysis characteristics, product composition and kinetics of co-pyrolysis of municipal solid waste (M) and paper sludge (P) with MgO additive were analyzed by TG-FTIR and Py–GC/MS. The experiment showed that, after adding paper sludge and MgO, there was a significant reduction in emission of pollutant and activation energy. The study reported that 70M30P (v/v) was the appropriate blend ratio with MgO. Co-pyrolysis characteristics were further studied by combining municipal solid waste and paper sludge under CO2 and N2 atmospheres. The studies inferred that the mass residue was lesser and pyrolysis index was higher in CO2 atmosphere than in the N2 atmosphere. The pyrolysis index of the blends improved with MgO catalyst (Fang et al. 2021).
The pyrolysis process was split into four stages for 90M10P and three steps for 70M30P and 50M50P. The activation energy of the blends at 110–600 °C is 170 kJ/mol and at 600–1000 °C is 300 kJ/mol. Increasing paper sludge percentage increased the average activation energy from 200 to 250 kJ/mol. Finally, the experiment was concluded that the temperature of 600 ºC was the boundary and 50M50P was the most suitable for treating sludge.
An experimental study was conducted using oil palm waste and paper sludge using thermogravimetric analysis to investigate the kinetics and co-pyrolysis characteristics of blends ratio from 10 to 90 wt%. The kinetic studies showed that the average activation energy does not decrease with increase in the proportion of oil palm waste. The observed lower activation energy was 152 kJ/mol by Starink method and 149 kJ/mol by Friedman method for 70% oil palm waste (Lin et al. 2014).
Thermochemical treatment of plastic waste
Plastic waste generation increases daily which get piled up in large volumes and merely end up in landfills. The degradation properties and degradation mechanisms of plastic wastes are highly complex to assess the feasibility, reactor design and scale-up process (Al Rayaan 2021). The influence of used and unused plastics refuse-derived fuel in the pilot-scale downdraft type fixed bed gasifier was studied. The results showed that both used and unused plastic waste showed the same yield of syngas but the higher heating value of unused plastic (781 kJ/Nm3) was relatively higher than the used plastics (500 kJ/Nm3) (Kungkajit et al. 2015).
The pyrolysis of waste plastic stream from industry and household plastic waste was investigated using microwave reactor made of quartz at operating frequency of 25 GHz under N2 atmosphere with carbon as microwave adsorbent. The pyrolytic products were bio-oil and biochar. The liquid product was quite viscous with certain fraction of aromatic compounds which can be used as a solvent and precursor. The surface morphology and amorphous nature of the solid product showed that the solid could be used for tar reduction (Aishwarya and Sindhu 2016).
High impact polystyrene plastics were investigated for gasification characteristics using supercritical water at a reaction temperature of 500–800 °C, reaction time 1–60 min, pressure 22–25 MPa and feedstock concentration 2–10 wt%. The experimental studies confirmed that 94.48 wt% carbon conversion rate was achieved at 800 °C using 3 wt% feedstock concentrations in a reaction time of 60 min at pressure 23 MPa and also reported that the change in pressure showed a minor change in gasification efficiency of plastics. The analysis of solid residue revealed the depolymerization of plastics and further proceeding of gasification reaction yielded carbon microspheres with a diameter of 0.8–1.5 µm (Bai et al. 2019).
Baby diapers are manufactured by using non-biodegradable plastics and super absorbent polymers which make them difficult to deteriorate. The microwave-assisted pyrolysis of used diapers was investigated for the influence of microwave power and operating temperature. The microwave-assisted pyrolysis process produced liquid oil (43 wt%), gases (29 wt%) and char (28 wt%). Alkanes, alkenes and esters were yielded in the liquid oil which has applications for chemical additives and cosmetics other than fuels. The high carbon, less nitrogen and zero sulfur contents of the char have a potential usage as soil additives and adsorption agent (Lam et al. 2019).
In recent times, due to coronavirus pandemic, about 54,000 tons per day (Nov 22, 2020 records) of medical wastes, viz., face mask, goggles and sanitizer container are reported. The management of the coronavirus disease-related medical wastes made of plastics using various thermochemical treatments has been studied widely. Incineration has practical application for all type of such wastes but also has a potential to emit hazardous gases. Gasification and pyrolysis are favorable for medical wastes considering the energy conversion efficiency and impacts on environment (Purnomo et al. 2021).
Thermochemical treatment of mixed municipal solid waste
Most often wastes are kept together without separating them into different components. Because of the complexity of municipal solid waste, any researchers studied the pyrolysis behavior of mixed municipal solid waste.
Wet torrefaction was considered as the best pre-treatment process for mixed municipal solid waste. Wet torrefaction experiments were conducted using leaf litter (34.67%), food waste (23.33%), vegetable waste (14.33%), fruit waste (11%) and non-recycled plastics (16.67%). The effect of wet torrefaction was studied at various temperatures (150, 175, 200 and 225 °C) in a 2.5 L stirring reactor. 200 °C temperature, holding time of 30 min and solid load of 1:2.5 were recommended as optimum conditions for the energy yield of 89%. The solid product from wet torrefaction process yielded a higher heating value of 33.01 MJ/kg (Triyono et al. 2019).
The influence of particle size and temperature on pyrolysis and gasification of municipal solid waste was studied in the laboratory-scale fixed bed reactor in the temperature range of 600–900 °C. The moisture content of samples was reduced to 10.2% and approximately 20 kg of municipal solid waste was used as feedstock. Particle size fraction below 5 mm (33.4 wt%), 5–10 mm (40.1 wt%) and 10 mm (26.5 wt%) were used. The study inferred that minimizing the size of particle improved the quality of gas in both pyrolysis and gasification. Higher temperature yielded more gas with less tar and char (Luo et al. 2010).
The influence of temperature was studied on mixture of municipal solid waste including cardboard, paper, plastic, vegetable waste, rubber and textile in a laboratory-scale fixed bed vacuum reactor by two pyrolysis process: isothermal and non-isothermal. About 500 g of municipal solid waste was loaded into the reactor for both the processes with the temperature maintained at 400 °C in case of isothermal process. The quality of bio-oil was higher in non-isothermal process when compared to the isothermal process. The bio-oil generated from isothermal process had high acid content which decreased the calorific value. However, the study reported that the product from non-isothermal process showed traces of impurities. The pre-treatment and upgradation are required to make bio-oil as a clean fuel (Gandidi et al. 2017).
Slow pyrolysis experiments were conducted using unsegregated municipal solid waste which includes plastics, yard waste, food waste, paper, textile, rubber, plastic sludge and household wastes. The wastes were volatized in four different temperature zones between 200 and 520 °C. Above 520 °C, a reduction in the quantity of bio-oil was observed. In general, the pyrolysis oil evidenced better ratio of aromatics to alkanes and low acidity oil mixture compared to non-interactive model and expected to raise by enhancing the ratio of rubber-biomass and rubber-plastic (Chhabra et al. 2020).
Investigations on the pyrolytic characteristics and kinetic behavior of mixed solid waste including yard waste, food waste, textile waste, paper, rubber, low-density polyethylene, high-density polyethylene, polypropylene, polyethylene terephthalate and polystyrene were carried out. Considering the complexity of municipal solid waste, the temperature for pyrolysis ranged between 170 and 520 °C. The biomass contents decomposed till 480 °C with the yield of char between 8 and 40 wt%. The plastic wastes got decomposed between 300 and 480 °C yielding 1 to 8 wt% of char. However, rubber samples produced large amount of char (41 wt%) and rubber samples decomposed in the range of 332 to 520 °C (Chhabra et al. 2019).
Six typical municipal solid waste which includes tire rubber, recycled polyvinyl chloride pellets, wood sawdust, paper mixture, kitchen waste and textile were assorted and analyzed under CO2 and N2 atmosphere during thermal degradation behavior using FTIR spectrometer. The author reported that similar effects were noted on pyrolysis of several waste materials under N2 and CO2 atmosphere. CO2 behaves inert at less than 600 °C and reacts after 600 °C. High temperature pyrolysis at CO2 atmosphere enhances char cracking and increases syngas production and reduces the amount of char (Tang et al. 2017).
Reforming
Reforming of biomass resources is recently becoming the popular research in bioenergy field. This section focuses on steam reforming, thermo-catalytic reforming and supercritical reforming. Production of tar is a common problem due to incomplete gasification of biomass into syngas. Tar causes severe problem such as clogging, loss in energy conversion efficiency because of the aromatic mixtures present in the tar. Tar contains polycyclic aromatic hydrocarbons which is hazardous to the environment when disposed in water resources.
Tar elimination can be accomplished using physical method, catalytic method and thermal cracking. To eliminate tar before downstream process, the stream is treated within the gasifier which is known as primary process or treated in a separate unit which is known as secondary process (Ashok et al. 2020). Steam reforming shows high efficiency in the production of hydrogen and also a simple process which is widely employed in refineries and fertilizer industries. The stoichiometry for steam reforming is given as
Heat requirement is considered as a main constraint in steam reforming to shift the equilibrium toward H2 and CO yield (Nahar et al. 2017). The different methods involved in steam reforming process are: (i) non-catalytic steam reforming which operates at a high temperature (1000–1400 °C) and (ii) catalytic steam reforming which is carried out at a low temperature (500–800 °C) (Remón et al. 2016).
Thermo-catalytic reforming is an enhanced intermediate pyrolysis process consisting of a 400–500 °C pyrolysis stage and a reforming stage. Thermo-catalytic reforming process produces H2 enriched syngas, biochar for soil remediation and high-quality bio-oil suitable for hydrodeoxygenation process (Neumann et al. 2015). The utilization of wide variety of biomasses (high humidity and high ash content) was considered as a major advantage in thermo-catalytic reforming process. Coking is an undesirable side reaction that degrades the activity of catalyst and mechanical strength. Coking can be reduced by optimizing the operating parameters such as temperature, steam to hydrocarbon ratio, space velocity (Huang et al. 2021a).
Bio-oil, syngas and biochar are generated during the reforming process. Nearly 30–45% of the biomass is converted to syngas, 7–15% is converted into bio-oil and 25–50% becomes biochar while water is generated as a by-product. Product quality and yield depend on the feedstock biomass composition. Thermo-catalytic reforming conversion process requires both thermal and electrical energy and also utilizes a part of the conversion product toward self-sustainability of energy potentially which lead to economic advantage (Moreno et al. 2020).
The thermo-catalysis of agricultural waste under different reforming temperatures reported that the increment in temperature (500–700 °C) causes adverse effects on properties of the product such as chemical composition, quality and quantity; higher reforming temperature yielded product with high heating value and H2 content (Santos et al. 2020).
Supercritical water reforming is a promising technology which converts wet biomass and organic wastes into hydrogen and syngas without vaporizing water. The critical temperature (Tc) and critical pressure (Pc) of water at supercritical condition are 647 K and 22.1 MPa, respectively, which is used to produce gas mixtures. Water in supercritical condition will convert biomass into pure hydrogen instead of pressurized steam. When compared with other reforming processes, supercritical water reforming process incurs less energy. The limitation of H2 solubility is reduced by employing supercritical water (Wongsakulphasatch et al. 2013).
The reforming of acetic acid, acetol, glucose and butanol under supercritical water conditions, supported by Ni-based catalyst were studied and the H2 yield under different parameters was discussed. The research conclusions were high temperature and low space velocity favored the H2 yield (Ortiz et al. 2018).
The production of gaseous and liquid biofuels from lignocellulosic biomass under sub-critical and supercritical water conditions have been studied and reported. The operating conditions, viz., temperature, reaction time, catalyst to bio-oil ratio and water content, showed significant influence on the product yield. Biogas was produced at high temperature and long reaction time while upgraded bio-oil was produced at low reaction time (Remón et al. 2016).
Catalysts used in the reforming process
The most significant challenge in the reforming process is selecting better catalyst; role of catalyst deactivation due to coke deposition and sintering makes the selection quite difficult. Catalysts can be classified into several types: metal-based (or synthetic), natural occurring and mineral catalyst. Catalytic activity is comparatively less in mineral catalysts when compared to natural and metal-based catalysts. Naturally occurring catalysts show higher tolerance to impurities such as H2S and HCl and are also easily replaceable in case of deactivation due to deposition of carbon. Table 3 represents the different types of catalyst and support material with varying temperature range for reforming process.
Calcined dolomite and zeolite catalyst, when trialed in downstream fixed bed reactor at 200–750 °C, showed that the former increased the gas yield and decreased the oil and char yield compared to zeolite catalyst. High temperature (600–750 °C) exhibited maximum gas yield and CO yield (Tursunov 2014).
Three catalysts such as olivine, dolomite and metal-based were studied for reforming 40 g/Nm3 acetic acid (a primary tar compound) by gasification of biomass using an updraft gasifier. The reforming process was done for 72 h in the temperature range 680–750 °C with the gas composition: H2O—35%, CO—2.3%, CO2—19.5%, CH4—3.6%, H2—24% and N2—15.6% by volume. The results revealed that metal-based catalyst showed good catalytic activity toward reverse water gas shift reaction while dolomite and olivine showed minor activity. Both dolomite and olivine did not show activity toward methane reforming. Olivine converted acetic acid with considerable amount of carbon deposit and the activity decreased over time, while carbon deposition occurred in dolomite at low temperatures. The metal catalyst completely converted acetic acid with almost no carbon deposition (Cavalli et al. 2021).
Ni-based catalysts are mostly used in large-scale industrial applications because of their ease of availability, low cost and high specific reactivity. The only disadvantage of using Ni catalyst is deactivation due to deposition of coke. Concerning the coke deposition problem, magnesium aluminate spinel which has high thermal conductivity and optimal surface property was used to mitigate the deposition. The effects of potassium as an alkaline promoter on MgAl2O4-supported Ni catalyst was investigated for CO2 reforming of methane. The chemical reactions reforming process using catalyst is shown (Fig. 3). Ni–K catalyst was prepared by wet co-impregnation onto MgAl2O4 and inferred that potassium concentrations of 3–5 wt% showed more efficiency toward CO2 reforming of methane (Azancot et al. 2021).
La-based perovskite (La0.6Sr0.2Co0.2Fe0.8O3-δ) (LSCF)-supported Ni-catalyst was prepared and studied for steam reforming of tar using toluene and phenol; the consortium was prepared by two different methods: Ruthenium embedded wetness impregnation and one-pot sol–gel method to produce LSCF-supported Ru Ni catalyst and Ni-LSCF catalysts, respectively. The resistance to deposition of coke and catalytic activity was witnessed for Ni-LSCF than LSCF-supported Ru Ni catalyst. LSCF-supported Ru Ni catalyst was reported as a good catalyst against coke formation during toluene reforming when compared to that when produced during phenol reforming by LSCF-supported Ni catalyst (Jurado et al. 2021).
Ni-based catalyst supported on Al2O3 and SiO2 was experimented in tubular fixed bed reactor using bio-oil aqueous phase. The experiment was carried out using supercritical water under different operating conditions (240 bar; 500–800 °C). The physical properties of catalyst changed under supercritical water condition at high temperature. The number of exposed metal atoms normalized the catalytic activity, leading to high turnover rates. When the reactant concentration increased, turnover frequency and space velocity increased (Ortiz et al. 2018).
The autothermal reforming of methane using nickel supported on perovskite as a catalytic agent was investigated. The catalyst was prepared by wetness impregnation method with CaTiO3, SrTiO3, BaTiO3 and Al2O3 as supports and characterized through energy dispersive X-ray spectroscopy and the results showed that CaTiO3-supported Ni catalyst and BaTiO3-supported Ni catalyst were considered as best catalysts based on activity and stability with over 70% conversion. Low conversion (50%) was noted in SrTiO3-supported Ni catalyst due to the deposition of carbon on the catalyst surface (Araújo et al. 2021).
Recently, biochar-based catalysts are gaining attention on account of their ease of major availability, easy functionalization, low cost and adaptability. Physically activated wheat straw-derived biochar under CO2 atmosphere at 700 °C temperature and 1 MPa pressure was studied. The biochar was utilized as a support material for metal-based catalyst. Ni performed better in steam reforming of acetic acid among five metallic active phases (Ni, Co, K, Ce and Fe). Moreover, Ni (10 wt%) and Co (7 wt%) showed positive results in acetic acid conversion, resistance to deactivation and stability.
The bimetallic Co–Ni-based catalyst was used for steam reforming of bio-oil containing water, acetone, ethanol, acetic acid and eugenol; catalyst deactivation prevailed within few minutes and conversion decreased from 50 to 30% due to the catalytic poisoning caused by decomposition of eugenol. The bimetallic catalyst displayed good efficiency during steam reforming at 750 °C, longer stability and high carbon conversion of 65% (Di Stasi et al. 2021).
Reforming for hydrogen production
The production of hydrogen can either be from fossil fuel or renewable resources and can be produced through several methods like water electrolysis and fuel steam reforming. At present, industries produce H2 through steam reforming of methane. About 95% of H2 is produced from fossil fuels, 4% from water and 1% from biomass.
The advances on glycerol steam reforming for enhanced hydrogen production using multifunctional reactors such as membrane reactor with H2 removal, sorption enhanced reactors with CO2 removal and other sorption enhanced membrane reactors were reported by several investigators. Apart from catalyst selection, the author also discusses on thermodynamic limitation and gave an alternative to combine glycerol steam reforming with CO2 or H2 removal in the same physical unit. Overall, the multifunctional reactor technology could help in the environmental perspective by capturing CO2 (Macedo et al. 2021).
The investigation of Rh-based catalyst supported on γ-Al2O3 and modified with CeO2, MgO or La2O3 in steam reforming of glycerol was attempted. The catalyst and support were synthesized by wet impregnation and surfactant (cetyltrimethylammonium bromide) assisted co-precipitation, respectively. The experiment was carried out in a continuous flow fixed bed reactor at 20:1 water-to-glycerol ratio (molar) at 400–750 °C and atmospheric pressure with space velocity of 50,000 ml/g hr. At 9:1 water-to-glycerol feed ratio, approximately 90% of total glycerol got converted with 78% H2 selectivity (Charisiou et al. 2020).
Exhaust gas fuel reforming for H2 production was studied in a catalytic fixed bed reactor using Al2O3-supported Ni catalyst at different wall temperatures. High wall temperature favored higher H2 concentration. CH4/O2 and H2O/CH4 ratio lesser than 1.5 resulted in lower H2 yield. The maximum H2 yield and hydrogen volume ratio reached up to 96% and 22%, respectively, at CH4/O2 and H2O/CH4 ratio is equal to 2. Enriched hydrogen production is also possible by absorption or adsorption of CO2 gas (Huang et al. 2021b).
NiCex Al (x is equal to 0.1, 0.3, 0.5, 0.7 and 0.9) mixed metal oxide catalyst was used for H2 production via steam reforming of glycerol. Ni is majorly used in cleaving C–C and C–H bond. The catalyst was synthesized by thermal decomposition and the experiment was carried out in conventional fixed bed reactor under atmospheric pressure. The experiment was concluded that NiCe7Al acted as the best catalyst with 89.2% gas conversion and 82.9% hydrogen selectivity (Jing et al. 2020).
Hydrogen production by removing CO2 using sorbents via sorption enhanced steam reforming process was studied. CaO-based sorbents capture CO2 at 550–800 °C, alkali metal ceramics at 550–600 °C and hydrotalcite sorbents at less than or equal to 550 °C. Enhanced H2 production can be achieved by catalyst modification: CO2 capture was enhanced by doping K2CO3 and other salts on alkali metal catalyst; and more than 90% of H2 production was achieved by incorporating metal oxide components to hydrotalcite catalyst. The production of hydrogen using sorbent increases the demand to produce sorbents from wastes (Wang et al. 2021b).
The steam reforming and partial oxidation of methane for H2 production under dielectric barrier discharge was carried out and studied the effects of H2O/CH4 and O2/N2 molar ratio, total gas flow, discharge voltage, discharge frequency and analyzed through in situ diagnostic emission spectroscopy. The study concluded that the hydrogen production increases with increasing molar ratios and discharge voltage but decreases with total gas flow rate. The conversion of methane and yield of H2 was observed as 47.45% and 21.33%, respectively, under the condition of 1.82 H2O/CH4, 2.1 O2/N2, 136 mL/min of total flow rate, 18.6 kV of discharge voltage and 9.8 kHz of discharge frequency (Feng et al. 2021).
Recent advances in production of H2 via discharge plasma reforming of methane in liquid phase were examined. The effect of microwave power and flow rate of methane gas was studied and the optimum methane conversion rate was reported as 94.3% at 900 W and resulted in 74% of hydrogen concentration. Besides, the highest efficiency of H2 production was obtained as approximately 0.92 mmol/kJ through improving the stability of plasma system (Wang et al. 2021a).
Effect of operating conditions and parameters on thermochemical treatment
The composition of the product yielded by thermochemical treatment methods is highly influenced not only by the feedstock properties but also by several operating parameters. The operating parameters include temperature, particle size, residence time, heating rate and reactor type. The effect of these variables on the municipal solid waste treatment to produce energy-oriented products reported by several investigators is reviewed in this section.
Temperature
Temperature plays a vital role in performance of thermal treatment and the composition of product changes significantly with temperature. An experimental study on combustion and co-combustion of raw and torrefied poultry litter waste was examined with different heating rates and different temperature conditions, viz., 250, 280 and 300 °C. The increase in temperature resulted with decrease in energy yield percentage from 102.47 to 90.96% (Atimtay et al. 2020).
The effect of temperature was studied on the tar yield from gasification of coconut and palm kernel shells using downdraft fixed bed gasifier in the range of 700–900 °C. The result showed that the tar yield and tar concentration decreased with increase in temperature. The higher heating value at 700–900 °C for coconut shells and palm kernel shells was in the range of 27.87–25.93 MJ/kg and 29.16–26.74 MJ/kg, respectively (Yahaya et al. 2020).
The characteristics of fuel from food waste were studied in the temperature range 150–600 °C in a horizontal tubular reactor. The study concluded the optimum temperature range for food waste between 290 and 330 °C and showed the energy and mass yield of the food waste decreased with an increase in torrefaction temperature (Poudel et al. 2015).
The effect of temperature in gasification of municipal solid waste was studied using a circulating fluidized bed gasifier. In the range of 500–650 °C, char gasification and steam reforming were restricted and increasing temperature range from 650 to 900 °C led to maximum H2 production. Moreover, maximum carbon conversion efficiency and cold gas efficiency were in the range of 40.1–79.90% and 29.90–88.90%, respectively (Shehzad et al. 2016).
The microwave torrefaction temperature profile of construction demolition waste and grass clipping at the microwave power levels (250, 500 and 750 W) was investigated and concluded that heating rate differs along the length of the reactor. In microwave torrefaction, the central part of reactor had the highest heating rate. Softwood pellets were gasified in a fluidized bed reactor at 0.23 kg/h feed rate with 1.3 kW power input to produce biofuel (Iroba et al. 2017).
Sugarcane bagasse and oat hulls were studied under different reforming temperatures in a thermo-catalytic reforming reactor. The reforming temperature range was between 500 and 700 °C. The article concluded that the bio-oil from thermo-catalytic reforming process contained the higher calorific value of 34.9 and 35.0 MJ/kg for both biomasses and obtained higher H2 yield (Santos et al. 2020). The generation of tar content was highly influenced by changing the temperature. 50 °C increment from 750 °C reduced tar production by half and 50 °C decrement doubled the amount of tar production (von Berg et al. 2021).
Thermal treatment of municipal solid waste was done in a fixed bed reactor and was inferred that temperature influences the char and tar yield of dry gas product. Studies show that by raising temperature from 600 to 900 °C, the gas yield can be maximized whereas the char and tar yield got reduced (Luo et al. 2010).
Particle size
The sizes of solid wastes are generally not uniformly distributed and need to be brought to uniformity and optimum size for best reactor outputs. The smaller size of particles offers higher surface area and particles get actively involved in enhanced heat and mass transfer (Mishra and Upadhyay 2021). A mixture of agricultural, forestry and industrial wastes was processed and crushed into particles of prescribed sizes. The reduction in size of particles enhanced the product quality. Particle size of 0.5 mm gasified in an entrained flow gasifier resulted in maximum fuel conversion (91.4%) (Hernández et al. 2010).
The effect of particle size on dry gas yield was studied with three municipal solid waste feeds of different size regimes, viz., less than 5 mm, 5–10 mm and 10–20 mm in a fixed bed reactor. Smaller particle size resulted in faster heating rates apparently for two reasons. One is the availability of larger effective heat transfer surface area during thermal treatment and the other was gas diffusion augmented reaction kinetics. The H2 and CO content increased from 18.3 to 22.4% and from 22 to 26.5%, respectively, with reduction in particle size (Luo et al. 2010).
Heating rate
Heating rate highly influences the performance of all thermal and thermochemical processes, particularly the yield of char, calorific value of fuels-derived and tar products during pyrolysis. An experiment was performed to study the effect of pyrolysis heating rate using plant biomass. Pyrolysis was carried out at 500 °C and 765 °C in the heating rate of 0.5 °C/s and 180 °C/s, respectively. Faster heating rate was found to decrease char yield and increase the yield of tar and total volatile content (Safdari et al. 2019).
The physical structure of char formed by devolatilization of bituminous coal and lignite was observed to be a function of heating rate; the high heating rate of lignite produced char with comparatively high internal surface area. The high heating rate favored devolatilization of low ranked coal and biofuel production thereby (Liu et al. 2020).
The influence of pyrolysis heating rate was studied at four different temperatures (450, 488, 525 and 600 °C) at the rate of 15 °C/min and 180 °C/min with waste polypropylene plastics in a bench-scale reactor under atmospheric and vacuum condition. The total yield of condensable products was maximum (93%) at 525 °C and slow heating rate in vacuum condition. Lower heating value was observed at high temperature due to the formation of aromatic compounds. The experiment was concluded to use pyrolysis under vacuum if diesel compounds are targeted as the fuel product (Parku et al. 2020).
An investigation on combustion and co-combustion characteristics of raw and torrefied poultry litter and blends of raw and torrefied poultry waste with lignite was studied at the heating rate of 5, 10, 20 and 30 °C/min. The lower heating rates showed effective heat transfer in the particle, while high heating rates significantly increased the combustion performance indices. Among the samples, raw poultry litter resulted in better combustion reactivity and torrefied poultry litter sample yielded high heating value from 13,932 to 18,903 kJ/kg with increasing torrefaction temperature (Atimtay et al. 2020).
Reactor type
Choice of reactor depends on feedstock (physical and chemical parameters), size of production and operating parameters. Operating conditions in each reactor may vary depending on the chemical nature and physical properties of feedstock (Raheem et al. 2020). A few prospective reactor types reported by researchers are discussed in this section.
The gasification study of coconut shells and palm kernel shells using downdraft fixed bed reactor showed the effects of temperature and characteristics of tar produced. The study confirmed that downdraft gasifier to be the most suitable one for combined heat and power electricity generation because the downdraft gasifier minimizes tar content in the product (Yahaya et al. 2020).
The different interactions between char and tar model were studied in fluidized bed reactor during gasification with steam. The interaction between char and tar yielded showed increased H2 production. But the catalyst (dolomite) used in steam gasification process showed low attrition resistance and was considered as inappropriate for fluidized bed reactor (Morin et al. 2018). A novel membrane film reactor was developed for biogas upgrading and liquid chemical production and achieved hydrogen conversion efficiency to the maximum of 98.1% (Zhao et al. 2020).
A novel continuous membrane reactor was developed with microporous TiO2/Al2O3 membrane. The reactor was packed with potassium hydroxide catalyst supported on palm shell biochar for bio-diesel production. The result showed that 94% bio-diesel conversion was achieved in the reactor at 70 °C (Baroutian et al. 2011). Microwave reactor was examined by many researchers on account of clean production and thermal efficiency of the biofuel (Phongprueksathat et al. 2019). In addition, microwave reactor showed certain limitations such as controlling microwave power and temperature in order to reduce the process repeatability (Raheem et al. 2020).
Residence time
Higher residence times offer more time of contact with the feedstocks, catalyst and other materials involved in the treatment. However, the resultant decrease in velocity during continuous operations may have inhibiting effects. The mean residence time of biomass conversion in an air blown bubbling fluidized bed gasification has been studied. The devolatilization and extinction time were measured. The study concluded that both values decreased with increasing feed rate and increasing air flow rate. With increasing residence time, the efficiency of gasification process increases to a particular extent (Agu et al. 2019).
Longer space residence time inside the entrained glow gasifier for the treatment of agricultural, forestry and industrial wastes achieved by low air flowrate enhanced the CO/H2 content, old gas efficiency and fuel conversion (Hernández et al. 2010). The increase in residence time during selective non-catalytic reduction in a laboratory-scale laminar flow reactor reduced the NOx emission and decreases the amount of undesirable pollutants and increases the amount of biofuel that can be achieved (Liang et al. 2014).
Techno-economic aspects of thermochemical treatments
Techno-economic analysis is used to estimate the cost and profitability of the system and investigate the feasibility in both technical and economic perspectives. Further, sensitivity analysis is performed to point out the main factors which influence the economic value and uncertainty. The supercritical water gasification (SCWG) process was economically analyzed for high energy conversion efficiency and environmental benefits. The pre-treatment processes like drying can be eliminated because supercritical water gasification can directly handle wet materials.
The factors affecting economic benefits of supercritical water gasification process are operation condition, system capacity and yield. The supercritical water gasification process inferred that increase in system capacity, feedstock concentration and reaction temperature can help in reducing hydrogen production cost. The average hydrogen production cost was calculated as 3.80 $/kg from various supercritical water gasification processes. When compared with other hydrogen production techniques, the H2 production cost from supercritical water gasification processes is fairly low (Chen et al. 2020a).
The economic feasibility of the supercritical water gasification process for combined heat and power production and H2 production from black liquor using stainless steel 316 and Inconel 625 as reactor materials was analyzed. The cost of energy produced from flue gas and that of raw materials were considered as major expenses. The study inferred Inconel reactor to be cost-effective than stainless steel. Further studies are required toward scale-up process due to the uncertainty of effects of catalysts on the reactor material (Özdenkçi et al. 2019).
Municipal solid waste fired combined cycle plant for energy production and the unit cost of electricity as the economic characteristic of the plant was estimated. The net electricity of about 3 MW per day with annual production of 12,500 MW was observed along with 44% overall efficiency. The small-scale off-grid plant was found to be beneficial for sustainable energy generation from municipal solid waste with expected unit cost of electricity to be INR 8/kWh (0.1235 $/kWh) (Mondal 2021).
Gasification-based chemical recycling, direct incineration and indirect incineration for municipal solid waste treatment were compared in an economic perspective. In comparison with incineration method, chemical recycling curbed greenhouse gas emission effectively but chemical recycling requires higher fixed capital investment. The study suggested that the economic performance of chemical recycling can be improved by integrating multiple treatments in a single large-scale chemical recycling plant (Voss et al. 2021).
The experimental study for bio-heavy oil production operated with 100 tons per day of sewage sludge in super and sub-critical water conditions and economic feasibility of the process was examined. The total capital investment of bio-heavy oil production was $15.1 million and $14.3 million using super and sub-critical water, respectively. The lower capital and production cost was noted in sub-critical water condition and considered as more economical. The work analyzed the total production cost as $2.1 million per yr. for both bio-heavy oil production variants; the net minimum fuel selling price of two plants was approximately 0.91$/L where the actual selling price was 0.55$/L.
The return on investment was higher in sub-critical water (6.6% per yr.) when compared to supercritical water (5.7% per yr.). Sensitivity analysis was also performed and showed that sewage sludge treatment had a great economic impact than bio-heavy oil price. The experiment concluded that bio-heavy oil production with sub-critical water was profitable than when carried out with supercritical water condition (Do et al. 2020).
The economic performance of waste valorization using thermochemical reforming was analyzed. Two agricultural wastes, olive wood pruning and digestate were used for energy conversion. The results showed that high-quality biochar production was a major benefit of the thermochemical reforming process. The study showed that the best performance can be obtained only if combined heat and power units were installed (Moreno et al. 2020).
The economic feasibility of integrated pyrolysis and combined heat and power unit with the organic fraction of municipal solid waste was reported. The pilot-scale tests showed that a plant operating with 5 tons per hr. feed flowrate can generate 4.4 MW of electricity and 5.3 MW of thermal energy with 27.2% overall electrical efficiency and 59.7% combined heat and power efficiency. The capital investment and leveled cost of electricity for this laboratory-scale plant was estimated to be £27.64 million and £0.063/kWh. Sensitivity analysis indicated that the most influential factors in this work were feedstock cost, electric power, enhancing the plant availability and productivity of fuel and reducing equipment cost (Yang et al. 2018).
The comparison study was conducted between fluidized bed gasifier at low temperature (870 °C) and entrained flow gasifier at elevated temperature (1300 °C) using corn stover as feedstock to estimate the capital and production costs. The results showed that the entrained flow gasifier requires extra investment when compared to the fluidized bed gasifier and the cost of product fuel was expected in the range of $4–5 per gallon. Sensitivity analysis showed that the feedstock purchase cost and total capital cost highly influenced the product value (Swanson et al. 2010).
The economic feasibility of the fluidized bed gasifier and entrained flow gasifiers for biomass gasification process was analyzed. The study reported that the economic efficiency of entrained flow gasifier was 11% higher than that of fluidized flow gasifier. In addition, the minimum fuel selling amount of the fluidized bed gasifier was found to be $0.3 per kg, which was lesser than that of the entrained flow gasifier (Salkuyeh et al. 2018).
Life cycle assessment and controlling emission from thermochemical treatments
Life cycle assessment is necessary to study the environmental impact and sustainability of biofuel production (Fig. 4). The life cycle assessment is focused on the reduction of greenhouse gas emission from biofuel production and includes the evaluation of emission factor, which is related to global warming potential, energy consumption, particulate matter emission, aquatic freshwater eutrophication and acidification. The discharge of pollutants such as NOx, SOx, CO2, CO and particulate matter from the thermochemical treatment of municipal solid waste for biofuel production was discussed in this section. The abatement of emission was examined by many researchers and most of the study showed that catalyst and municipal solid waste ratio played a vital role in emission control.
The life cycle assessment of catalytic gasification for H2 production using wheat straw as feedstock was studied. The process was divided into five units, namely, biomass collection and pre-treatment (P1), biochar catalyst preparation using fast pyrolysis (P2), two-stage pyrolysis–gasification unit (P3), product separation unit (P4) and H2 distribution to downstream plant (P5). The study showed that the product separation unit (P4) was found to generate higher greenhouse gas and resource depletion impacts (Loy et al. 2021).
The valorization of bio-oil using supercritical reforming followed by low temperature Fischer–Tropsch process was studied. The life cycle assessment studies were performed in the three case studies chosen in a comparative way (with or without CO2 storage). They were (i) complete reforming of natural gas to H2, (ii) reforming partial fraction of aqueous phase and (iii) aqueous phase reforming to H2. Out of these, the second case study with CO2 storage showed the lowest global warming potential impact and reported the significant reduction of emission with respect to fossil fuel (Ortiz et al. 2020).
The investigation on an innovative management of waste electrical and electronic plastics which includes treatments such as sorting, dissolution or precipitation, extrusion, catalytic pyrolysis and plastic upgrading was conducted. The life cycle assessment showed that this method could enhance the environmental performance by improving 580%, 60% and 17% of global warming impact, carcinogenic impact and non-renewable energy impacts, respectively, and also inferred that the waste management is sustainable only if waste exportation and improper treatment is reduced (Ardolino et al. 2021).
The life cycle assessment for torrefied pellets using rice husk in four different temperatures and torrefaction medium was investigated. The torrefaction process was carried out in inert medium at 240 °C, 30 min (case 1), inert medium at 300 °C, 30 min (case 2), partially oxidative medium at 240 °C, 30 min (case 3) and partially oxidative medium at 300 °C, 30 min (case 4). Among these, case 4 yielded the best life cycle assessment report followed by case 3 in the context of process and environmental oriented impacts (Thengane et al. 2020).
The life cycle assessment studies for solar-based hydrogen production in oil and gas industries were conducted. The study compared two fossil fuel-based such as steam methane reforming and coal gasification and two solar based such as photovoltaic and solar thermal electrolysis hydrogen production pathways. The result stated that the total greenhouse gas emissions were 10.28, 11.59, 3.08, 2.06 kg CO2/kg H2 for steam methane reforming, coal gasification, photovoltaic electrolysis and solar thermal electrolysis, respectively. The greenhouse gas emission was higher for construction of solar plant than for hydrogen production units (Sadeghi et al. 2020).
The influence of municipal solid waste ratio (0, 20 and 40 wt%) on all emissions was presented for electricity power generation from co-gasification of pelletized municipal solid waste and chopped switchgrass in certain ratios. The result showed that CO, NOx and CO2 emissions decreased, at the same time hydrocarbons and SOx emissions increased with increasing municipal solid waste ratio. In addition, the author evaluated the generation of emission for five different engine loads (1, 2, 3, 4 and 5 kW) and reported that CO, NOx, CO2 and SOx decreased with increasing load except hydrocarbons (Indrawan et al. 2018).
Particulate emission from gasification and pyrolysis of biomass under 500, 600 and 700 °C was studied. The particles emitted from gasification and pyrolysis process were mainly under the size range of 0.25–1 µm and 1–2.5 µm, respectively. The result showed that the highest proportion of particles in the emission were ranging from 0.25 to 1 µm due to their size-dependent volume concentration, which is about 39.60%, 51.94%, 58.14%, 43.08%, 53.16%, 65.29% for 500 °C gasification, 600 °C gasification, 700 °C gasification, 500 °C pyrolysis, 600 °C pyrolysis, 700 °C pyrolysis, respectively (Yao et al. 2018).
The influence of mixed waste ratio on emission was studied using five different wastes such as domestic garbage, sludge and swill waste along with coal and grass biomass in circulating fluidized bed at 850 °C. The result reported that the NOx emission was reduced by adjusting the ratio and the dioxin emission was lower than the emission standard. However, the emission of mercury, lead and combinations of chromium, tin, antimony, copper and manganese were significantly high and exceeded the pollution control standard (Zhang et al. 2015).
The toxic polychlorinated dibenzo-p-dioxins and dibenzofurans were found in emission from torrefaction process of municipal solid waste, refused solid waste and construction and demolition wood from batch-scale reactor at 220 °C and residence time of 90 min. The result showed that the municipal solid waste—construction and demolition waste emit lower toxic matter than residual-derived fuel blends (Edo et al. 2017).
The cytotoxicity of fine particles emitted from municipal solid waste and biomass combustion was examined. Fine particles with diameter less than 2.5 µm from municipal solid waste and biomass combustion was subjected to cytotoxic test on human adenocarcinoma alveolar basal epithelial cells (A549) and normal human bronchial epithelial cells (BEAS-2B) and reported that municipal solid waste emitted significantly higher content of heavy metals (Pb, Zn and Cu) and dioxins than biomass, which caused most severe cell injury on BEAS-2B cells than A549 (Shang et al. 2019).
The impact of changes in pre-treatment of rice straw combustion on particulate matter size range 1–10 was investigated. The author introduced the combination of water washing, viz., water washing after torrefaction (W–T), torrefaction after water washing (T–W) and hydrothermal carbonization under certain conditions such as oxy-fuel condition (oxy30 and oxy50), long leaching time (24 h), large H2O to biomass ratio (40:1). The result showed that W–T ratio (1:40) was suitable for particulate matter size PM1 emission under oxy50 condition and W–T ratio (1:10) was suitable for PM10 emission under both combustion condition for practical application (PM: particulate matter) (Wang et al. 2020).
The co-combustion of rice husk and coal was carried on drop tube furnace at 1300 °C and studied the formation of PM10 (particle size 10 µm) from combustion of raw rice husk, torrefied rice husk and blends with lignite. The result showed that the co-combustion of coal and torrefied rice husk emitted less PM1 but more PM1-10 than co-combustion with raw rice husk (Han et al. 2019).
Nitrous oxide emission from household waste, viz., food and non-food waste under different O2 concentrations (21%, 10% and 1%) was studied. The result showed that the N2O emission of food waste was decreased with decrease in O2 concentration. On the contrary, the N2O emission of non-food waste increased with decrease in O2 concentration. The study concluded that the non-food waste showed maximum N2O emission when compared to food waste (Feng et al. 2020).
The greenhouse gas emission of biochar obtained from Mangifera indica seeds and Passiflora edulis shells via torrefaction process at 210–300 °C was investigated and compared the emission performance with coal. The result showed that greenhouse gas emission from obtained biochar was significantly low when compared with combustion of coal and co-firing 10–20% of biochar and coal effectively mitigated the greenhouse gas emission (Lin et al. 2021).
Plastic waste was subjected to laboratory-scale pyrolysis process at 500 ± 30 °C with and without catalyst (fly ash and zeolite). The catalyst to feed ratio was 1:10. The engine test showed that using blend fuel (80:20; diesel: plastic pyrolytic oil) reduced oxides of nitrogen and hydrocarbon emission, when compared to pure diesel or pure pyrolytic oil (Singh et al. 2020).
The emission characteristics of pyrolytic oil obtained from mixed plastic waste using Fe2O3 doped Al2O3 were examined. Pyrolytic oil to diesel fuel ratio (25:75) with 25 ppm of Al2O3 was tested by using as fuel in water cooled diesel engine and resulted that the addition of Al2O3 effectively reduced the hydrocarbon and CO2 emission. In addition, NOx emission reduced than pure pyrolytic oil and diesel fuel (Sekar et al. 2021).
The emission of CO, NOx and particulate matter from paper briquettes along with wood briquettes, coal briquettes and kindling firewood was investigated. The result showed that higher CO concentration was obtained from paper and coal briquettes. Furthermore, paper briquettes emitted the highest NOx level. The burning of paper briquettes and wood generated particulate matter with diameter of 72 and 68 nm than coal and kindling firewood (45 and 51 nm). Overall, the study reported that using paper briquettes for domestic heating purpose will not cause severe exposure of particulate matter, CO and NOx (Xiu et al. 2018).
Perspective
The design of reactors for continuous processes and capital cost are the major challenges in commercializing the production of biofuel via thermochemical treatments of municipal solid waste which could withstand high temperature, high pressure, attrition of catalysts and feedstock biomass. Free radical production is one of the important demerits in co-gasification process and the mechanism is still unknown due to the lack of detection methods of free radicals. Research studies to explore free radical issue will be of greater scope in alleviating the limitation of co-gasification processes and make the process prospective on commercial scales. Additionally, studies are needed to integrate two or more thermochemical treatments to give a better energy output. The integration could be torrefaction with pyrolysis, torrefaction with gasification, anaerobic digestion with gasification. The integrative approaches are modeled based on the influence of feedstock composition, optimum operating conditions required, techno-economic and sensitivity analysis.
Furthermore, life cycle assessment studies are required for the waste valorization via integrated pathways. Microwave-assisted and plasma-assisted treatments were stated in various research works, but microwave-assisted are hindered by a lack of understanding in industrial microwave heating applications. However, plasma-assisted solution has been proven as the best method for disposing municipal solid waste and should be made accessible and available at suitable prices by innovative design in municipal corporations.
Liquid fuel obtained via pyrolysis process is considered as the best alternative, but the fuel has to meet the government standards prior to commercial usage. Investigations on the fabrication of novel catalysts, promoters and support materials to enhance the selectivity, productivity and activity with no or little effects on coking, sintering and poisoning can be carried out. Determining the degradation pathways using computational and modeling approaches to generate fuel and energy from the municipal solid wastes in eco-friendly and energy efficient operations can bring an insight to build technologies on this domain that can cater human needs.
Conclusion
The step-by-step implementation and proper management of municipal solid waste generation are obligatory at local, national and global levels to have a pollutant free environment. Thermochemical treatment for municipal solid waste has been considered as one of the most promising technologies to convert solid waste into useful and profitable products. This review presents the various thermochemical treatments for municipal solid waste and reforming methods for enhanced biofuel products and reports pertaining to the emission of undesirable products and their impacts on environment are reviewed. The limitations and pragmatic challenges were alleviated by several modifications in the existing technology for practical implementation. Heterogeneous catalysts were used in several works for better catalytic properties yielding promising potential than homogeneous catalysts. Catalysts used in reforming processes for H2 production and life cycle assessments were briefly reviewed.
Abbreviations
- TGA–FTIR:
-
Thermogravimetric analysis–Fourier transform infrared spectroscopy
- Py–GC/MS:
-
Pyrolysis–gas chromatography/mass spectroscopy
- SCWG:
-
Supercritical water gasification
References
Acharya B, Dutta A, Minaret J (2015) Review on comparative study of dry and wet torrefaction. Sustain Energy Technol Assess 12:26–37. https://doi.org/10.1016/j.seta.2015.08.003
Agar D, Wihersaari M (2012) Bio-coal, torrefied lignocellulosic resources–key properties for its use in co-firing with fossil coal–their status. Biomass Bioenerg 44:107–111. https://doi.org/10.1016/j.biombioe.2012.05.004
Agu CE, Pfeifer C, Eikeland M, Tokheim LA, Moldestad BM (2019) Measurement and characterization of biomass mean residence time in an air-blown bubbling fluidized bed gasification reactor. Fuel 253:1414–1423. https://doi.org/10.1016/j.fuel.2019.05.103
Aishwarya KN, Sindhu N (2016) Microwave assisted pyrolysis of plastic waste. Procedia Technol 25:990–997. https://doi.org/10.1016/j.protcy.2016.08.197
Al Rayaan MB (2021) Recent advancements of thermochemical conversion of plastic waste to biofuel-a review. Clean Eng Technol. https://doi.org/10.1016/j.clet.2021.100062
Angulo-Mosquera LS, Alvarado-Alvarado AA, Rivas-Arrieta MJ, Cattaneo CR, Rene ER, García-Depraect O (2021) Production of solid biofuels from organic waste in developing countries: a review from sustainability and economic feasibility perspectives. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.148816
Araújo PM, da Costa KM, Passos FB (2021) Hydrogen production from methane autothermal reforming over CaTiO3, BaTiO3 and SrTiO3 supported nickel catalysts. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2021.04.202
Ardolino F, Cardamone GF, Arena U (2021) How to enhance the environmental sustainability of WEEE plastics management: an LCA study. Waste Manage 135:347–359. https://doi.org/10.1016/j.wasman.2021.09.021
Arena U (2012) Process and technological aspects of municipal solid waste gasification: a review. Waste Manage 32(4):625–639. https://doi.org/10.1016/j.wasman.2011.09.025
Ashok J, Dewangan N, Das S, Hongmanorom P, Wai MH, Tomishige K, Kawi S (2020) Recent progress in the development of catalysts for steam reforming of biomass tar model reaction. Fuel Process Technol 199:106252. https://doi.org/10.1016/j.fuproc.2019.106252
Atimtay A, Yurdakul S (2020) Combustion and Co-Combustion characteristics of torrefied poultry litter with lignite. Renew Energy 148:1292–1301. https://doi.org/10.1016/j.renene.2019.10.068
Azancot L, Bobadilla LF, Centeno MA, Odriozola JA (2021) Effect of potassium loading on basic properties of Ni/MgAl2O4 catalyst for CO2 reforming of methane. J. CO2 Utilization 52:101681. https://doi.org/10.1016/j.jcou.2021.101681
Bada SO, Falcon RMS, Falcon LM (2014) Investigation of combustion and co-combustion characteristics of raw and thermal treated bamboo with thermal gravimetric analysis. Thermochim Acta 589:207–214. https://doi.org/10.1016/j.tca.2014.05.021
Bai B, Liu Y, Wang Q, Zou J, Zhang H, Jin H, Li X (2019) Experimental investigation on gasification characteristics of plastic wastes in supercritical water. Renew Energy 135:32–40. https://doi.org/10.1016/j.renene.2018.11.092
Bandara JC, Jaiswal R, Nielsen HK, Moldestad BM, Eikeland MS (2021) Air gasification of wood chips, wood pellets and grass pellets in a bubbling fluidized bed reactor. Energy. https://doi.org/10.1016/j.energy.2021.121149
Baroutian S, Aroua MK, Raman AAA, Sulaiman NM (2011) A packed bed membrane reactor for production of biodiesel using activated carbon supported catalyst. Biores Technol 102(2):1095–1102. https://doi.org/10.1016/j.biortech.2010.08.076
Basagiannis AC, Verykios XE (2006) Reforming reactions of acetic acid on nickel catalysts over a wide temperature range. Appl Catal A 308:182–193. https://doi.org/10.1016/j.apcata.2006.04.024
Cai M, Guy C, Héroux M, Lichtfouse E, An C (2021) The impact of successive COVID-19 lockdowns on people mobility, lockdown efficiency, and municipal waste solid. Environ Chem Lett 19(6):3959–3965. https://doi.org/10.1007/s10311-021-01290-z
Cavalli A, Tetteroo R, Graziadio M, Aravind PV (2021) Catalytic reforming of acetic acid as main primary tar compound from biomass updraft gasifiers: screening of suitable catalysts and operating conditions. Biomass Bioenerg 146:105982. https://doi.org/10.1016/j.biombioe.2021.105982
Chan WP, Veksha A, Lei J, Oh WD, Dou X, Giannis A, Lisaka G, Lim TT (2019) A hot syngas purification system integrated with downdraft gasification of municipal solid waste. Appl Energy 237:227–240. https://doi.org/10.1016/j.apenergy.2019.01.031
Charisiou ND, Italiano C, Pino L, Sebastian V, Vita A, Goula MA (2020) Hydrogen production via steam reforming of glycerol over Rh/γ-Al2O3 catalysts modified with CeO2, MgO or La2O3. Renew Energy 162:908–925. https://doi.org/10.1016/j.renene.2020.08.037
Chen WH, Peng J, Bi XT (2015) A state-of-the-art review of biomass torrefaction, densification and applications. Renew Sustain Energy Rev 44:847–866. https://doi.org/10.1016/j.rser.2014.12.039
Chen J, Ma X, Yu Z, Deng T, Chen X, Chen L, Dai M (2019) A study on catalytic co-pyrolysis of kitchen waste with tire waste over ZSM-5 using TG-FTIR and Py GC/MS. Biores Technol 289:121585. https://doi.org/10.1016/j.biortech.2019.121585
Chen J, Liang J, Xu Z, Jiaqiang E (2020a) Assessment of supercritical water gasification process for combustible gas production from thermodynamic, environmental and techno-economic perspectives: a review. Energy Convers Manage 226:113497. https://doi.org/10.1016/j.enconman.2020.113497
Chen L, Wen C, Wang W, Liu T, Liu E, Liu H, Li Z (2020b) Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renew Energy 161:867–877. https://doi.org/10.1016/j.renene.2020.06.148
Chen WH, Lin BJ, Lin YY, Chu YS, Ubando AT, Show PL, Ong HC, Chang JS, Ho SH, Culaba AB, Pétrissans A, Pétrissans M (2021) Progress in biomass torrefaction: principles, applications and challenges. Prog Energy Combust Sci 82:100887. https://doi.org/10.1016/j.pecs.2020.100887
Chhabra V, Bhattacharya S, Shastri Y (2019) Pyrolysis of mixed municipal solid waste: characterisation, interaction effect and kinetic modelling using the thermogravimetric approach. Waste Manage 90:152–167. https://doi.org/10.1016/j.wasman.2019.03.048
Chhabra V, Bambery K, Bhattacharya S, Shastri Y (2020) Thermal and in situ infrared analysis to characterise the slow pyrolysis of mixed municipal solid waste (MSW) and its components. Renew Energy 148:388–401. https://doi.org/10.1016/j.renene.2019.10.045
Couto N, Silva V, Rouboa A (2016) Municipal solid waste gasification in semi-industrial conditions using air-CO2 mixtures. Energy 104:42–52. https://doi.org/10.1016/j.energy.2016.03.088
Di Stasi C, Cortese M, Greco G, Renda S, González B, Palma V, Manyà JJ (2021) Optimization of the operating conditions for steam reforming of slow pyrolysis oil over an activated biochar-supported Ni–Co catalyst. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2021.05.193
Do TX, Mujahid R, Lim HS, Kim JK, Lim YI, Kim J (2020) Techno-economic analysis of bio heavy-oil production from sewage sludge using supercritical and subcritical water. Renew Energy 151:30–42. https://doi.org/10.1016/j.renene.2019.10.138
Edo M, Skoglund N, Gao Q, Persson PE, Jansson S (2017) Fate of metals and emissions of organic pollutants from torrefaction of waste wood, MSW, and RDF. Waste Manage 68:646–652. https://doi.org/10.1016/j.wasman.2017.06.017
Fang S, Yu Z, Lin Y, Lin Y, Fan Y, Liao Y, Ma X (2017a) A study on experimental characteristic of co-pyrolysis of municipal solid waste and paper mill sludge with additives. Appl Therm Eng 111:292–300. https://doi.org/10.1016/j.applthermaleng.2016.09.102
Fang S, Yu Z, Ma X, Lin Y, Lin Y, Chen L, Fan Y, Liao Y (2017b) Co-pyrolysis characters between combustible solid waste and paper mill sludge by TG-FTIR and Py-GC/MS. Energy Convers Manage 144:114–122. https://doi.org/10.1016/j.enconman.2017.04.046
Fang S, Lin Y, Huang Z, Huang H, Chen S, Ding L (2021) Investigation of co-pyrolysis characteristics and kinetics of municipal solid waste and paper sludge through TG-FTIR and DAEM. Thermochim Acta 700:178889. https://doi.org/10.1016/j.tca.2021.178889
Feng H, Wang X, Cai J, Chen S (2020) Discrepancies in N2O emissions between household waste and its food waste and non-food waste components during the predisposal stage. J Environ Manage 265:110548. https://doi.org/10.1016/j.jenvman.2020.110548
Feng XU, Wang YM, Fan LI, Nie XY, Zhu LH (2021) Hydrogen production by the steam reforming and partial oxidation of methane under the dielectric barrier discharge. J Fuel Chem Technol 49(3):367–373. https://doi.org/10.1016/S18725813(21)60022-1
Gaeta-Bernardi A, Parente V (2016) Organic municipal solid waste (MSW) as feedstock for biodiesel production: a financial feasibility analysis. Renew Energy 86:1422–1432. https://doi.org/10.1016/j.renene.2015.08.025
Gandidi IM, Susila MD, Pambudi NA (2017) Production of valuable pyrolytic oils from mixed municipal solid waste (MSW) in Indonesia using non-isothermal and isothermal experimental. Case Stud Therm Eng 10:357–361. https://doi.org/10.1016/j.csite.2017.08.003
Grycová B, Koutník I, Pryszcz A (2016) Pyrolysis process for the treatment of food waste. Biores Technol 218:1203–1207. https://doi.org/10.1016/j.biortech.2016.07.064
Gunarathne V, Ashiq A, Ramanayaka S, Wijekoon P, Vithanage M (2019) Biochar from municipal solid waste for resource recovery and pollution remediation. Environ Chem Lett 17(3):1225–1235. https://doi.org/10.1007/s10311-019-00866-0
Guo F, Zhao X, Peng K, Liang S, Jia X, Qian L (2019) Catalytic reforming of biomass primary tar from pyrolysis over waste steel slag-based catalysts. Int J Hydrogen Energy 44(31):16224–16233. https://doi.org/10.1016/j.ijhydene.2019.04.190
Gupta N, Yadav KK, Kumar V (2015) A review on current status of municipal solid waste management in India. J Environ Sci 37:206–217. https://doi.org/10.1016/j.jes.2015.01.034
Han J, Yu D, Yu X, Liu F, Wu J, Zeng X, Yu G, Xu M (2019) Effect of the torrefaction on the emission of PM10 from combustion of rice husk and its blends with a lignite. Proc Combust Inst 37(3):2733–2740. https://doi.org/10.1016/j.proci.2018.07.011
Hasan MM, Rasul MG, Khan MMK, Ashwath N, Jahirul MI (2021) Energy recovery from municipal solid waste using pyrolysis technology: a review on current status and developments. Renew Sustain Energy Rev 145:111073. https://doi.org/10.1016/j.rser.2021.111073
Hernández JJ, Aranda-Almansa G, Bula A (2010) Gasification of biomass wastes in an entrained flow gasifier: effect of the particle size and the residence time. Fuel Process Technol 91(6):681–692. https://doi.org/10.1016/j.fuproc.2010.01.018
Huang J, Veksha A, Chan WP, Lisak G (2021a) Support effects on thermocatalytic pyrolysis-reforming of polyethylene over impregnated Ni catalysts. Appl Catal A. https://doi.org/10.1016/j.apcata.2021.118222
Huang Y, Zhang Z, Wei W, Long Y, Li G (2021b) Experimental study on characteristics of hydrogen production from exhaust gas-fuel reforming in a catalytic fixed-bed reactor. Fuel 290:120068. https://doi.org/10.1016/j.fuel.2020.120068
Indrawan N, Thapa S, Bhoi PR, Huhnke RL, Kumar A (2018) Electricity power generation from co-gasification of municipal solid wastes and biomass: generation and emission performance. Energy 162:764–775. https://doi.org/10.1016/j.energy.2018.07.169
Iroba KL, Baik OD, Tabil LG (2017) Torrefaction of biomass from municipal solid waste fractions I: temperature profiles, moisture content, energy consumption, mass yield, and thermochemical properties. Biomass Bioenergy 105:320–330. https://doi.org/10.1016/j.biombioe.2017.07.009
Jaideep R, Lo WH, Lim GP, Chua CX, Gan S, Lee LY, Thangalazhy-Gopakumar S (2021) Enhancement of fuel properties of yard waste through dry torrefaction. Mater Sci Energy Technol 4:156–165. https://doi.org/10.1016/j.mset.2021.04.001
Jing F, Liu S, Wang R, Li X, Yan Z, Luo S, Chu W (2020) Hydrogen production through glycerol steam reforming over the NiCexAl catalysts. Renew Energy 158:192–201. https://doi.org/10.1016/j.renene.2020.05.044
Jurado L, Papaefthimiou V, Thomas S, Roger AC (2021) Upgrading syngas from wood gasification through steam reforming of tars over highly active Ni-perovskite catalysts at relatively low temperature. Appl Catal B 299:120687. https://doi.org/10.1016/j.apcatb.2021.120687
Kan T, Strezov V, Evans T, He J, Kumar R, Lu Q (2020) Catalytic pyrolysis of lignocellulosic biomass: a review of variations in process factors and system structure. Renew Sustain Energy Rev 134:110305. https://doi.org/10.1016/j.rser.2020.110305
Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA (2007) Sustainable hydrogen production via reforming of ethylene glycol using a novel spouted bed reactor. Catal Today 127(1–4):246–255. https://doi.org/10.1016/j.cattod.2007.05.018
Khan MZH, Sultana M, Al-Mamun MR, Hasan MR (2016) Pyrolytic waste plastic oil and its diesel blend: fuel characterization. J Environ Public Health. https://doi.org/10.1155/2016/7869080
Kungkajit C, Prateepchaikul G, Kaosol T (2015) Influence of plastic waste for refuse-derived fuel on downdraft gasification. Energy Procedia 79:528–535. https://doi.org/10.1016/j.egypro.2015.11.529
Lam SS, Mahari WAW, Ma NL, Azwar E, Kwon EE, Peng W, Chong CT, Liu Z, Park YK (2019) Microwave pyrolysis valorization of used baby diaper. Chemosphere 230:294–302. https://doi.org/10.1016/j.chemosphere.2019.05.054
Lasek JA, Głód K, Słowik K (2021) The co-combustion of torrefied municipal solid waste and coal in bubbling fluidised bed combustor under atmospheric and elevated pressure. Renew Energy 179:828–841. https://doi.org/10.1016/j.renene.2021.07.106
Liang L, Hui S, Pan S, Shang T, Liu C, Wang D (2014) Influence of mixing, oxygen and residence time on the SNCR process. Fuel 120:38–45. https://doi.org/10.1016/j.fuel.2013.11.050
Lin YL, Zheng NY (2021) Torrefaction of fruit waste seed and shells for biofuel production with reduced CO2 emission. Energy 225:120226. https://doi.org/10.1016/j.energy.2021.120226
Lin Y, Ma X, Yu Z, Cao Y (2014) Investigation on thermochemical behavior of co-pyrolysis between oil-palm solid wastes and paper sludge. Biores Technol 166:444–450. https://doi.org/10.1016/j.biortech.2014.05.101
Liu S, Niu Y, Wen L, Kang Y, Wang D (2020) Effects of physical structure of high heating-rate chars on combustion characteristics. Fuel 266:117059. https://doi.org/10.1016/j.fuel.2020.117059
Loy ACM, Alhazmi H, Lock SSM, Yiin CL, Cheah KW, Chin BLF, How BF, Yusup S (2021) Life-cycle assessment of hydrogen production via catalytic gasification of wheat straw in the presence of straw derived biochar catalyst. Biores Technol 341:125796. https://doi.org/10.1016/j.biortech.2021.125796
Luo S, Xiao B, Hu Z, Liu S, Guan Y, Cai L (2010) Influence of particle size on pyrolysis and gasification performance of municipal solid waste in a fixed bed reactor. Biores Technol 101(16):6517–6520. https://doi.org/10.1016/j.biortech.2010.03.060
Macedo MS, Soria MA, Madeira LM (2021) Process intensification for hydrogen production through glycerol steam reforming. Renew Sustain Energy Rev 146:111151. https://doi.org/10.1016/j.rser.2021.111151
Madhiyanon T, Sathitruangsak P, Soponronnarit S (2010) Combustion characteristics of rice-husk in a short-combustion-chamber fluidized-bed combustor (SFBC). Appl Therm Eng 30(4):347–353. https://doi.org/10.1016/j.applthermaleng.2009.09.014
Mamvura TA, Danha G (2020) Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 6(3):e03531. https://doi.org/10.1016/j.heliyon.2020.e03531
Mishra S, Upadhyay RK (2021) Review on biomass gasification: gasifiers, gasifying mediums, and operational parameters. Mater Sci Energy Technol. https://doi.org/10.1016/j.mset.2021.08.009
Mondal P (2021) Techno-economic and environmental performance assessment of a MSW to energy plant for Indian urban sectors. Therm Sci Eng Progress 21:100777. https://doi.org/10.1016/j.tsep.2020.100777
Monisha RS, Mani RL, Sivaprakash B, Rajamohan N, Vo DVN (2021) Green remediation of pharmaceutical wastes using biochar: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01348-y
Moreno VC, Iervolino G, Tugnoli A, Cozzani V (2020) Techno-economic and environmental sustainability of biomass waste conversion based on thermocatalytic reforming. Waste Manage 101:106–115. https://doi.org/10.1016/j.wasman.2019.10.002
Morin M, Nitsch X, Hémati M (2018) Interactions between char and tar during the steam gasification in a fluidized bed reactor. Fuel 224:600–609. https://doi.org/10.1016/j.fuel.2018.03.050
Nahar G, Mote D, Dupont V (2017) Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew Sustain Energy Rev 76:1032–1052. https://doi.org/10.1016/j.rser.2017.02.031
Nanda S, Berruti F (2021) Thermochemical conversion of plastic waste to fuels: a review. Environ Chem Lett 19(1):123–148. https://doi.org/10.1007/s10311-020-01094-7
Neumann J, Binder S, Apfelbacher A, Gasson JR, García PR, Hornung A (2015) Production and characterization of a new quality pyrolysis oil, char and syngas from digestate–Introducing the thermo-catalytic reforming process. J Anal Appl Pyrol 113:137–142. https://doi.org/10.1016/j.jaap.2014.11.022
Ong HC, Chen WH, Singh Y, Gan YY, Chen CY, Show PL (2020) A state-of-the-art review on thermochemical conversion of biomass for biofuel production: a TG-FTIR approach. Energy Convers Manage 209:112634. https://doi.org/10.1016/j.enconman.2020.112634
Ortiz FG, Campanario FJ (2018) Hydrogen production from supercritical water reforming of acetic acid, acetol, 1-butanol and glucose over Ni-based catalyst. J Supercrit Fluids 138:259–270. https://doi.org/10.1016/j.supflu.2018.04.023
Ortiz FG, Alonso-Fariñas B, Campanario FJ, Kruse A (2020) Life cycle assessment of the Fischer-Tropsch biofuels production by supercritical water reforming of the bio-oil aqueous phase. Energy 210:118648. https://doi.org/10.1016/j.energy.2020.118648
Özdenkçi K, De Blasio C, Sarwar G, Melin K, Koskinen J, Alopaeus V (2019) Techno-economic feasibility of supercritical water gasification of black liquor. Energy 189:116284. https://doi.org/10.1016/j.energy.2019.116284
Park J, Meng J, Lim KH, Rojas OJ, Park S (2013) Transformation of lignocellulosic biomass during torrefaction. J Anal Appl Pyrol 100:199–206. https://doi.org/10.1016/j.jaap.2012.12.024
Parku GK, Collard FX, Görgens JF (2020) Pyrolysis of waste polypropylene plastics for energy recovery: influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process Technol 209:106522. https://doi.org/10.1016/j.fuproc.2020.106522
Phongprueksathat N, Meeyoo V, Rirksomboon T (2019) Steam reforming of acetic acid for hydrogenproduction: catalytic performances of Ni and co supported on Ce0.75Zr0.25O2 catalysts. Int J Hydrog Energy 44(18):9359–9367. https://doi.org/10.1016/j.ijhydene.2019.02.085
Poudel J, Ohm TI, Oh SC (2015) A study on torrefaction of food waste. Fuel 140:275–281. https://doi.org/10.1016/j.fuel.2014.09.120
Purnomo CW, Kurniawan W, Aziz M (2021) Technological review on thermochemical conversion of COVID-19-related medical wastes. Resour Conserv Recycl. https://doi.org/10.1016/j.resconrec.2021.105429
Quan C, Xu S, Zhou C (2017) Steam reforming of bio-oil from coconut shell pyrolysis over Fe/olivine catalyst. Energy Convers Manage 141:40–47. https://doi.org/10.1016/j.enconman.2016.04.024
Raheem I, Mohiddin MNB, Tan YH, Kansedo J, Mubarak NM, Abdullah MO, Ibrahim ML (2020) A review on influence of reactor technologies and kinetic studies for biodiesel application. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2020.08.024
Rajamohan N, Sivaprakash B (2008) Biosorption of heavy metal using brown seaweed in a regenerable continuous column. Asia-Pac J Chem Eng 3(5):572–578. https://doi.org/10.1002/apj.202
Rajamohan N, Sivaprakash B (2010) Biosorption of Inorganic mercury onto marine alga Sargassum tenerrimum: batch and column studies. Int J Environ Technol Manage 12(2–4):229–239. https://doi.org/10.1504/IJETM.2010.031530
Rawoof SAA, Kumar PS, Vo DVN, Subramanian S (2021) Sequential production of hydrogen and methane by anaerobic digestion of organic wastes: a review. Environ Chem Lett 19(2):1043–1063. https://doi.org/10.1007/s10311-020-01122-6
Remón J, Arcelus-Arrillaga P, García L, Arauzo J (2016) Production of gaseous and liquid bio-fuels from the upgrading of lignocellulosic bio-oil in sub-and supercritical water: effect of operating conditions on the process. Energy Convers Manage 119:14–36. https://doi.org/10.1016/j.enconman.2016.04.010
Sadeghi S, Ghandehariun S, Rosen MA (2020) Comparative economic and life cycle assessment of solar-based hydrogen production for oil and gas industries. Energy 208:118347. https://doi.org/10.1016/j.energy.2020.118347
Safdari MS, Amini E, Weise DR, Fletcher TH (2019) Heating rate and temperature effects on pyrolysis products from live wildland fuels. Fuel 242:295–304. https://doi.org/10.1016/j.fuel.2019.01.040
Saidi M, Gohari MH, Ramezani AT (2020) Hydrogen production from waste gasification followed by membrane filtration: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01030-9
Salkuyeh YK, Saville BA, MacLean HL (2018) Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int J Hydrog Energy 43(20):9514–9528. https://doi.org/10.1016/j.ijhydene.2018.04.024
Sánchez A, Artola A, Font X, Gea T, Barrena R, Gabriel D, Monedero MAS, Roig A, Cayuela ML, Mondini C (2015) Greenhouse gas emissions from organic waste composting. Environ Chem Lett 13(3):223–238. https://doi.org/10.1007/s10311-015-0507-5
Santamaria L, Lopez G, Arregi A, Amutio M, Artetxe M, Bilbao J, Olazar M (2018) Influence of the support on Ni catalysts performance in the in-line steam reforming of biomass fast pyrolysis derived volatiles. Appl Catal B 229:105–113. https://doi.org/10.1016/j.apcatb.2018.02.003
Santos J, Ouadi M, Jahangiri H, Hornung A (2020) Thermochemical conversion of agricultural wastes applying different reforming temperatures. Fuel Process Technol 203:106402. https://doi.org/10.1016/j.fuproc.2020.106402
Savuto E, Di Carlo A, Gallucci K, Di Giuliano A, Rapagnà S (2020) Steam gasification of lignite and solid recovered fuel (SRF) in a bench scale fluidized bed gasifier. Waste Manage 114:341–350. https://doi.org/10.1016/j.wasman.2020.07.016
Sekar M, Praveenkumar TR, Dhinakaran V, Gunasekar P, Pugazhendhi A (2021) Combustion and emission characteristics of diesel engine fueled with nanocatalyst and pyrolysis oil produced from the solid plastic waste using screw reactor. J Clean Prod 318:128551. https://doi.org/10.1016/j.jclepro.2021.128551
Shahbaz M, AlNouss A, Ghiat I, Mckay G, Mackey H, Elkhalifa S, Al-Ansari T (2021) A comprehensive review of biomass based thermochemical conversion technologies integrated with CO2 capture and utilisation within BECCS networks. Resour Conserv Recycl 173:105734. https://doi.org/10.1016/j.resconrec.2021.105734
Shang Y, Wu M, Zhou J, Zhang X, Zhong Y, An J, Qian G (2019) Cytotoxicity comparison between fine particles emitted from the combustion of municipal solid waste and biomass. J Hazard Mater 367:316–324. https://doi.org/10.1016/j.jhazmat.2018.12.065
Shehzad A, Bashir MJ, Sethupathi S (2016) System analysis for synthesis gas (syngas) production in Pakistan from municipal solid waste gasification using a circulating fluidized bed gasifier. Renew Sustain Energy Rev 60:1302–1311. https://doi.org/10.1016/j.rser.2016.03.042
Sikarwar VS, Pohořelý M, Meers E, Skoblia S, Moško J, Jeremiáš M (2021) Potential of coupling anaerobic digestion with thermochemical technologies for waste valorization. Fuel 294:120533. https://doi.org/10.1016/j.fuel.2021.120533
Singh TS, Verma TN, Singh HN (2020) A lab scale waste to energy conversion study for pyrolysis of plastic with and without catalyst: engine emissions testing study. Fuel 277:118176. https://doi.org/10.1016/j.fuel.2020.118176
Sivaprakash B, Rajamohan N (2011) Equilibrium and kinetics studies on the biosorption of As (III) and As (V) by the Marine Algae Turbinaria conoides. Res J Environ Sci 5(10):779–789. https://doi.org/10.3923/rjes.2011.779.789
Smidt E, Meissl K, Tintner J, Ottner F (2010) Interferences of carbonate quantification in municipal solid waste incinerator bottom ash: evaluation of different methods. Environ Chem Lett 8(3):217–222. https://doi.org/10.1007/s10311-009-0209-y
Swanson RM, Platon A, Satrio JA, Brown RC (2010) Techno-economic analysis of biomass-to-liquids production based on gasification. Fuel 89:S11–S19. https://doi.org/10.1016/j.fuel.2010.07.027
Tang Y, Ma X, Lai Z, Zhou D, Lin H, Chen Y (2012) NOx and SO2 emissions from municipal solid waste (MSW) combustion in CO2/O2 atmosphere. Energy 40(1):300–306. https://doi.org/10.1016/j.energy.2012.01.070
Tang Y, Ma X, Wang Z, Wu Z, Yu Q (2017) A study of the thermal degradation of six typical municipal waste components in CO2 and N2 atmospheres using TGA-FTIR. Thermochim Acta 657:12–19. https://doi.org/10.1016/j.tca.2017.09.009
Tang Y, Huang Q, Sun K, Chi Y, Yan J (2018) Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Biores Technol 249:16–23. https://doi.org/10.1016/j.biortech.2017.09.210
Thengane SK, Burek J, Kung KS, Ghoniem AF, Sanchez DL (2020) Life cycle assessment of rice husk torrefaction and prospects for decentralized facilities at rice mills. J Clean Prod 275:123177. https://doi.org/10.1016/j.jclepro.2020.123177
Tran KQ, Luo X, Seisenbaeva G, Jirjis R (2013) Stump torrefaction for bioenergy application. Appl Energy 112:539–546. https://doi.org/10.1016/j.apenergy.2012.12.053
Triyono B, Prawisudha P, Aziz M, Pasek AD, Yoshikawa K (2019) Utilization of mixed organic-plastic municipal solid waste as renewable solid fuel employing wet torrefaction. Waste Manage 95:1–9. https://doi.org/10.1016/j.wasman.2019.05.055
Tursunov O (2014) A comparison of catalysts zeolite and calcined dolomite for gas production from pyrolysis of municipal solid waste (MSW). Ecol Eng 69:237–243. https://doi.org/10.1016/j.ecoleng.2014.04.004
Vamvuka D, Sfakiotakis S, Kotronakis M (2012) Fluidized bed combustion of residues from oranges’ plantations and processing. Renew Energy 44:231–237. https://doi.org/10.1016/j.renene.2012.01.083
Varol M, Atimtay AT, Olgun H, Atakül H (2014) Emission characteristics of co-combustion of a low calorie and high sulfur–lignite coal and woodchips in a circulating fluidized bed combustor: part 1. Effect of excess air ratio. Fuel 117:792–800. https://doi.org/10.1016/j.fuel.2013.09.051
von Berg L, Pongratz G, Pilatov A, Almuina-Villar H, Hochenauer C, Scharler R, Anca-Couce A (2021) Correlations between tar content and permanent gases as well as reactor temperature in a lab-scale fluidized bed biomass gasifier applying different feedstock and operating conditions. Fuel 305:121531. https://doi.org/10.1016/j.fuel.2021.121531
Voss R, Lee RP, Seidl L, Keller F, Fröhling M (2021) Global warming potential and economic performance of gasification-based chemical recycling and incineration pathways for residual municipal solid waste treatment in Germany. Waste Manage 134:206–219. https://doi.org/10.1016/j.wasman.2021.07.040
Wang W, Wen C, Liu T, Li C, Xu J, Liu E, Liua H, Yan K (2020) Emission reduction of PM10 via pretreatment combining water washing and carbonisation during rice straw combustion: focus on the effects of pretreatment and combustion conditions. Fuel Process Technol 205:106412. https://doi.org/10.1016/j.fuproc.2020.106412
Wang Q, Wang J, Zhu T, Zhu X, Sun B (2021a) Characteristics of methane wet reforming driven by microwave plasma in liquid phase for hydrogen production. Int J Hydrog Energy 46(69):34105–34115. https://doi.org/10.1016/j.ijhydene.2021.08.006
Wang Y, Memon MZ, Seelro MA, Fu W, Gao Y, Dong Y, Ji G (2021b) A review of CO2 sorbents for promoting hydrogen production in the sorption-enhanced steam reforming process. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2021.01.206
Wongsakulphasatch S, Kiatkittipong W, Assabumrungrat S (2013) Comparative study of fuel gas production for SOFC from steam and supercritical-water reforming of bioethanol. Int J Hydrog Energy 38(14):5555–5562. https://doi.org/10.1016/j.ijhydene.2013.02.125
Xing Z, Ping Z, Xiqiang Z, Zhanlong S, Wenlong W, Jing S, Yanpeng M (2021) Applicability of municipal solid waste incineration (MSWI) system integrated with pre-drying or torrefaction for flue gas waste heat recovery. Energy 224:120157. https://doi.org/10.1016/j.energy.2021.120157
Xiu M, Stevanovic S, Rahman MM, Pourkhesalian AM, Morawska L, Thai PK (2018) Emissions of particulate matter, carbon monoxide and nitrogen oxides from the residential burning of waste paper briquettes and other fuels. Environ Res 167:536–543. https://doi.org/10.1016/j.envres.2018.08.008
Yahaya AZ, Somalu MR, Muchtar A, Sulaiman SA, Daud WRW (2020) Effects of temperature on the chemical composition of tars produced from the gasification of coconut and palm kernel shells using downdraft fixed-bed reactor. Fuel 265:116910. https://doi.org/10.1016/j.fuel.2019.116910
Yang Y, Wang J, Chong K, Bridgwater AV (2018) A techno-economic analysis of energy recovery from organic fraction of municipal solid waste (MSW) by an integrated intermediate pyrolysis and combined heat and power (CHP) plant. Energy Convers Manage 174:406–416. https://doi.org/10.1016/j.enconman.2018.08.033
Yang Y, Liew RK, Tamothran AM, Foong SY, Yek PNY, Chia PW, Tran TV, Peng W, Lam SS (2021) Gasification of refuse-derived fuel from municipal solid waste for energy production: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01177-5
Yao Z, You S, Dai Y, Wang CH (2018) Particulate emission from the gasification and pyrolysis of biomass: Concentration, size distributions, respiratory deposition-based control measure evaluation. Environ Pollut 242:1108–1118. https://doi.org/10.1016/j.envpol.2018.07.126
Zhang H, Lv C, Li J, Qin W, Hu Y, Dong C (2015) Solid waste mixtures combustion in a circulating fluidized bed: emission properties of NOx, dioxin and heavy metals. Energy Procedia 75:987–992. https://doi.org/10.1016/j.egypro.2015.07.322
Zhang LH, Chyang CS, Duan F, Li PW, Chen SY (2016) Comparison of the thermal behaviors and pollutant emissions of pelletized bamboo combustion in a fluidized bed combustor at different secondary gas injection modes. Energy 116:306–316. https://doi.org/10.1016/j.energy.2016.09.116
Zhang Z, Hu X, Li J, Gao G, Dong D, Westerhof R, Hu S, Xiang J, Wang Y (2018) Steam reforming of acetic acid over Ni/Al2O3 catalysts: correlation of nickel loading with properties and catalytic behaviors of the catalysts. Fuel 217:389–403. https://doi.org/10.1016/j.fuel.2017.12.114
Zhang C, Hu X, Yu Z, Zhang Z, Chen G, Li C, Liu Q, Xiang J, Wang Y, Hu S (2019) Steam reforming of acetic acid for hydrogen production over attapulgite and alumina supported Ni catalysts: impacts of properties of supports on catalytic behaviors. Int J Hydrogen Energy 44(11):5230–5244. https://doi.org/10.1016/j.ijhydene.2018.09.071
Zhao L, Wang Z, Ren HY, Nan J, Chen C, Ren NQ (2020) Improving biogas upgrading and liquid chemicals production simultaneously by a membrane biofilm reactor. Biores Technol 313:123693. https://doi.org/10.1016/j.biortech.2020.123693
Zhou H, Long Y, Meng A, Li Q, Zhang Y (2015) Thermogravimetric characteristics of typical municipal solid waste fractions during co-pyrolysis. Waste Manage 38:194–200. https://doi.org/10.1016/j.wasman.2014.09.027
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nandhini, R., Berslin, D., Sivaprakash, B. et al. Thermochemical conversion of municipal solid waste into energy and hydrogen: a review. Environ Chem Lett 20, 1645–1669 (2022). https://doi.org/10.1007/s10311-022-01410-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01410-3