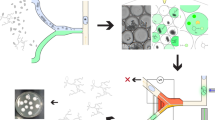
Abstract
The selection of improved producers among the huge number of variants in mutant libraries is a key issue in filamentous fungi of industrial biotechnology. Here, we developed a droplet-based microfluidic high-throughput screening platform for selection of high-cellulase producers from filamentous fungus Trichoderma reesei. The screening system used a fluorogenic assay to measure amount of cellulase and its activity. The key effectors such as cellulase-inducing medium, spore germination, droplet cultivation time, droplet fluorescence signal detection, and droplet cell sorting were studied. An artificial pre-mixed library of high- and low-cellulase-producing T. reesei strains was screened successfully to verify the feasibility of our method. Finally, two cellulase hyperproducers exhibiting improvements in cellulase activity of 27% and 46% were isolated from an atmospheric and room-temperature plasma (ARTP)-mutated library. This high-throughput screening system could be applied to the engineering of T. reesei strains and other industrially valuable protein-producing filamentous fungi.




Similar content being viewed by others
References
Abatemarco J, Sarhan MF, Wagner JM, Lin JL, Liu L, Hassouneh W, Yuan SF, Alper HS, Abate AR (2017) RNA-aptamers-in-droplets (RAPID) high-throughput screening for secretory phenotypes. Nat Commun 8:332
Aro N, Pakula T, Penttilä M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Baltz RH (1986) Mutagenesis in Streptomyces. In: Demain AL, Soloman NA (eds) Manual of industrial microbiology and biotechnology. American Society for Microbiology, Washington, DC, pp 184–190
Beneyton T, Wijaya IP, Postros P, Najah M, Leblond P, Couvent A, Mayot E, Griffiths AD, Drevelle A (2016) High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci Rep 6:27223
Cheng Y, Song X, Qin Y, Qu Y (2009) Genome shuffling improves production of cellulase by Penicillium decumbens JU-A10. J Appl Microbiol 107:1837–1846
Dillon AJ, Bettio M, Pozzan FG, Andrighetti T, Camassola M (2011) A new Penicillium echinulatum strain with faster cellulase secretion obtained using hydrogen peroxide mutagenesis and screening with 2-deoxyglucose. J Appl Microbiol 111:48–53
Farrell AE, Plevin RJ, Turner BT, Jones AD, O’Hare M, Kammen DM (2006) Ethanol can contribute to energy and environmental goals. Science 311:506–508
Gao F, Hao Z, Sun X, Qin L, Zhao T, Liu W, Luo H, Yao B, Su X (2018) A versatile system for fast screening and isolation of Trichoderma reesei cellulase hyperproducers based on DsRed and fluorescence-assisted cell sorting. Biotechnol Biofuels 11:261
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Gusakov AV (2011) Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol 29:419–425
He R, Li C, Feng J, Zhang D (2017) A class II hydrophobin gene, Trhfb3, participates in fungal asexual development of Trichoderma reesei. FEMS Microbiol Lett 1:364
He R, Li C, Ma L, Zhang D, Chen S (2016) Effect of highly branched hyphal morphology on the over-production of cellulase in Trichoderma reesei DES-15. 3 Biotech 6:214
Herpoël-Gimbert I, Margeot A, Dolla A, Jan G, Mollé D, Lignon S, Mathis H, Sigoillot JC, Monot F, Asther M (2008) Comparative secretome analyses of two Trichoderma reesei RUT-C30 and CL847 hypersecretory strains. Biotechnol Biofuels 23:18
Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW (2015) Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161:1187–1201
Korfer G, Pitzler C, Vojcic L, Martinez R, Schwaneberg U (2016) In vitro flow cytometry-based screening platform for cellulase engineering. Sci Rep 6:26128
Liu YT, Luo ZY, Long CN, Wang HD, Long MN, Hu Z (2011) Cellulase production in a new mutant strain of Penicillium decumbens ML-017 by solid state fermentation with rice bran. N Biotechnol 28:733–737
Ma LJ, Li C, Yang ZH, Jia WD, Zhang DY, Chen SL (2013) Kinetic studies on batch cultivation of Trichoderma reesei and application to enhance cellulase production by fed-batch fermentation. J Biotechnol 166:192–197
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EG, Grigoriev IV, Harris P, Jackson M, Kubicek CP, Han CS, Ho I, Larrondo LF, de Leon AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barabote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol 26:553–560
Mazutis L, Gilbert J, Ung WL, Weitz DA, Griffiths AD, Heyman JA (2013) Single-cell analysis and sorting using droplet-based microfluidics. Nat Protoc 8:870–891
Meng Q, Liu C, Zhao X, Bai F (2018) Engineering Trichoderma reesei Rut-C30 with the overexpression of egl1 at the ace1 locus to relieve repression on cellulase production and to adjust the ratio of cellulolytic enzymes for more efficient hydrolysis of lignocellulosic biomass. J Biotechnol 285:56–63
Montenecourt BS, Eveleigh DE (1977) Preparation of mutants of Trichoderma reesei with enhanced cellulase production. Appl Environ Microbiol 34:777–782
Morikawa Y, Ohashi T, Mantani O, Okada H (1995) Cellulase induction by lactose in Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol 44:106–111
Najah M, Calbrix R, Mahendra-Wijaya IP, Beneyton T, Griffiths AD, Drevelle A (2014) Droplet-based microfluidics platform for ultra-high-throughput bioprospecting of cellulolytic microorganisms. Chem Biol 21:1722–1732
Nevalainen H, Suominen P, Taimisto K (1994) On the safety of Trichoderma reesei. J Biotechnol 37:193–200
Obexer R, Godina A, Garrabou X, Mittl PR, Baker D, Griffiths AD, Hilvert D (2017) Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase. Nat Chem 9:50–56
Peberdy JF (1994) Protein secretion in filamentous fungi—trying to understand a highly productive black box. Trends Biotechnol 12:50–57
Peterson R, Nevalainen H (2012) Trichoderma reesei RUT-C30—thirty years of strain improvement. Microbiology 158:58–68
Pucher ME, Steiger MG, Mach RL, Mach-Aigner AR (2011) A modified expression of the major hydrolase activator in Hypocrea jecorina (Trichoderma reesei) changes enzymatic catalysis of biopolymer degradation. Catal Today 10:122–128
Qiao Y, Zhao X, Zhu J, Tu R, Dong L, Wang L, Dong Z, Wang Q, Du W (2018) Fluorescence-activated droplet sorting of lipolytic microorganisms using a compact optical system. Lab Chip 18:190–196
Shea JJ (1998) Surfactants and polymers in aqueous solutions. IEEE Electric Insul Mag 14:42–43
Sheldon RA (2014) Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem 16:950–963
Wang S, Liu G, Wang J, Yu J, Huang B, Xing M (2013) Enhancing cellulase production in Trichoderma reesei RUT C30 through combined manipulation of activating and repressing genes. J Ind Microbiol Biotechnol 40:633–641
Wang M, Zhao Q, Yang J, Jiang B, Wang F, Liu K, Fang X (2013) A mitogen-activated protein kinase Tmk3 participates in high osmolarity resistance, cell wall integrity maintenance and cellulase production regulation in Trichoderma reesei. PLoS One 8:e72189
Wang Y, Yan J (2002) Effect of surfactant on cellulase production by Trichoderma. Biotechnology 12:37–38 (in Chinese)
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20:295–299
Zhang F, Zhao X, Bai F (2018) Improvement of cellulase production in Trichoderma reesei Rut-C30 by overexpression of a novel regulatory gene Trvib-1. Bioresour Technol 247:676–683
Zou G, Shi S, Jiang Y, van den Brink J, de Vries RP, Chen L, Zhang J, Ma L, Wang C, Zhou Z (2012) Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering. Microb Cell Fact 8:21
Acknowledgements
We are grateful to Prof. David A. Weitz and Dr. Lloyd W. Ung (School of Engineering and Applied Science (SEAS), Harvard University) for valuable discussion on droplet microfluidic technology and the kind of providing microfluidic devices. This work was supported by the National Key R&D Program of China (2016YFD0501405) and the Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-SW-STS-165). Ran Tu is supported by China Scholarship Council (201404910306) during visiting studies at Harvard University. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN). CNS is part of Harvard University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Movie 1
Droplet generation. Capture rate: 3000 frames per second; replay rate: 5 frames per second (AVI 6940 kb)
Supplementary Movie 2
Droplet sorting. Droplets exhibiting strong fluorescence (i.e., high-cellulase activity) were sorted out. Capture rate: 800 frames per second; replay rate: 80 frames per second (MP4 10626 kb)
Rights and permissions
About this article
Cite this article
He, R., Ding, R., Heyman, J.A. et al. Ultra-high-throughput picoliter-droplet microfluidics screening of the industrial cellulase-producing filamentous fungus Trichoderma reesei. J Ind Microbiol Biotechnol 46, 1603–1610 (2019). https://doi.org/10.1007/s10295-019-02221-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02221-2