Abstract
Standardized reporting of multiparametric prostate MRI (mpMRI) is widespread and follows international standards (Pi-RADS). However, quantitative measurements from mpMRI are not widely comparable. Although T2 mapping sequences can provide repeatable quantitative image measurements and extract reliable imaging biomarkers from mpMRI, they are often time-consuming. We therefore investigated the value of quantitative measurements on a highly accelerated T2 mapping sequence, in order to establish a threshold to differentiate benign from malignant lesions. For this purpose, we evaluated a novel, highly accelerated T2 mapping research sequence that enables high-resolution image acquisition with short acquisition times in everyday clinical practice. In this retrospective single-center study, we included 54 patients with clinically indicated MRI of the prostate and biopsy-confirmed carcinoma (n = 37) or exclusion of carcinoma (n = 17). All patients had received a standard of care biopsy of the prostate, results of which were used to confirm or exclude presence of malignant lesions. We used the linear mixed-effects model-fit by REML to determine the difference between mean values of cancerous tissue and healthy tissue. We found good differentiation between malignant lesions and normal appearing tissue in the peripheral zone based on the mean T2 value. Specifically, the mean T2 value for tissue without malignant lesions was (151.7 ms [95% CI: 146.9–156.5 ms] compared to 80.9 ms for malignant lesions [95% CI: 67.9–79.1 ms]; p < 0.001). Based on this assessment, a limit of 109.2 ms is suggested. Aditionally, a significant correlation was observed between T2 values of the peripheral zone and PI-RADS scores (p = 0.0194). However, no correlation was found between the Gleason Score and the T2 relaxation time. Using REML, we found a difference of -82.7 ms in mean values between cancerous tissue and healthy tissue. We established a cut-off-value of 109.2 ms to accurately differentiate between malignant and non-malignant prostate regions. The addition of T2 mapping sequences to routine imaging could benefit automated lesion detection and facilitate contrast-free multiparametric MRI of the prostate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (pCA) is the second most common solid tumor in men worldwide and has the highest age-standardized rate (ASR) in Northern Europe at 83 per 100,000. The lifetime cumulative risk to develop a pCA is 3.9% [1].
Multiparametric MRI (mpMRI) is the preferred imaging modality for prostate cancer and includes T1-weighted, T2-weighted (T2w), diffusion-weighted (DWI), and dynamic contrast-enhanced (DCE) sequences [2, 3]. MpMRI complements digital rectal examination (DRE), serum-PSA measurement, transrectal ultrasound-guided (TRUS) in imaging. So far, the mpMRI has generally shown high sensitivity but low specificity in diagnosing pCA [4].
Standardized reporting among urologists, radiologists and oncologists is crucial for the diagnosis and treatment planning of pCA. Although widespread and well-established, scoring of prostate lesions using the Prostate Imaging-Reporting and Data System (PI-RADS) is subjective by nature. in particular, non-experienced radiologists may find it challenging to differentiate between tumor-free and tumor-containing tissue in the prostate [5]. However, standard mpMRI acquisitions do not regularly provide quantitative image measurements, resulting in subjective or semi-objective interpretation and poor reproducibility of quantitative measurements on follow-up examinations.
The T2w sequence is particularly relevant for anatomical assessment of the prostate. Local variations in radiofrequency inhomogeneities of the transmitting and receiving coil make T2w values generally suitable as a qualitative measure. T2 mapping (T2M) values, on the other hand, can be processed as a quantitative parameter since they are based on multiple echo times, and thus reflect the relaxation time of the protons independent of their relative position to the coil [3]. However, the unfavorable resolution to acquisition time ratio has impeded the wide-spread adoption of this technique into standard clinical protocols [3].
Previous investigations have shown that T2M-derived measurements can detect malignant prostate lesions in standardized mpMRI [3, 6,7,8]. In this analysis, we evaluated the value of the latest-generation quantitative MR data acquisition methods in detecting and characterizing prostate lesions in the peripheral zone [9]. A research application was utilized for fast T2M with high spatial resolution, supporting parallel imaging and model-based reconstruction [9]. The technical novelty of this technique allows for high acceleration, resulting in acquisition times that are acceptable in clinical routine.
We therefore evaluated the mean relaxation time of malignant lesions compared to benign lesions and tumor-free prostate regions in different age groups. Additionally, the study aimed to determine if radiologists could use the highly accelerated T2M sequence to more easily distinguish malignant from healthy tissue. The study derived a threshold value to help classify suspicious prostate lesions as malignant or non-malignant.
Material and Methods
Patients
We conducted a retrospective case-control study, approved by the Institutional Review Board (IRB No. 19-299). Cases were identified by an independent investigator by querying the hospital's Picture Archiving and Communication System (PACS) for prostate MRIs between 08/2018 and 07/2019. The study’s inclusion criteria required an examination to exclude prostate carcinoma. Patients who had received a T2M sequence as part of the mpMRI protocol with total coverage of the prostate volume during this period were included (acquisition parameters are included in the Supplementary material). Of 260 patients, 91 met these inclusion criteria (Fig. 1).
The data from the clinical database concerning the patients and their MRI were recorded and transferred to our study database (including the population parameters age, examination date, findings on mpMRI, PIRADS score) (Fig. 2).
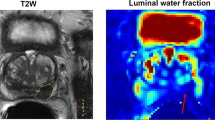
Confirmed prostate cancer of the right peripheral zone. Coregistration of the sections from T2M (A the color scale on the left image border is ranging from 0 to 200 ms), T2w (B), DWI b1000 (C) and ADC (D) sequences for one case of the pCAp group. The malignant lesion of the right peripheral zone is marked by an arrowhead in T2M where darker values correspond to lower relaxation times. In the corresponding region of the T2w decreased signal intensity can be observed in the posterolateral aspect of the right peripheral prostate zone (arrowhead). From ADC and DWI, a diffusion restriction is visible, demonstrating infiltration of the right transitional zone (arrow). The figure includes a color legend on the right border indicating relaxation times per voxel. Relaxation times larger than 200 ms are displayed in red
From the hospital information system, we recorded whether the patient had undergone previous surgery, biopsy, or ablation or embolisation of the prostate, as well as the presence of prostatitis. The final cohort included only patients who had not undergone any interventions on the prostate and had a prostate biopsy subsequent to mpMRI. Biopsy was defined as a systematic, standard-of-care biopsy. For these, pathology reports had to be available in the hospital data system and specify results per prostate region (n = 61).
The remaining group was further defined based on data quality criteria. Exclusion criteria included poor image quality due to relevant motion artifacts, incomplete or non-displayable T2M sequences, and unavailable or incomplete biopsy reports. Poor image quality was defined as not sufficiently interpretable by an experienced radiologist.
The final study group comprised 54 patients. Of these 37 patients were diagnosed with prostate carcinoma and for 17 cases the presence of prostate carcinoma was excluded. The mean age of the group was 65.5 years (minimum 42, maximum 83 years). Tables 1 and 2 list the median PIRADS scores.
Procedures and Techniques
Imaging
MRI imaging was performed for all patients at 3 Tesla (MAGNETOM PrismaFit, Siemens Healthineers, Erlangen, Germany).
The mpMRI protocol included T2w imaging in 3 orientations, Diffusion Weighted Imaging (DWI), dynamic contrast-enhanced T1-weighted (same orientation as axial T2-weighted and DWI) and pre-contrast T2M (0.7 × 0.7 × 3.0 mm3, 16 echoes with ΔTE of 10.8 ms, TR 5000 ms, acceleration factor = 10; Fig. 3). Image reconstruction parameters are listed in the Supplementary material. Notably, T2M, a research application sequence, “GRAPPATINI”, that accelerates a multi-echo spin-echo sequence is used to achieve acquisition times that are feasible in clinical routine [9]. Particularly in this study, a tenfold acceleration is achieved by combining a twofold GRAPPA acceleration with a fivefold model-based acceleration resulting in an acquisition time of 4:37 min [9, 10]. GRAPPATINI has been evaluated in various body parts including the knee, brain, spine, pancreas, cervix, and prostate [3, 6, 11,12,13,14,15]. The method has been compared to various other methods across organs [13, 16].
Example of region of interest (ROI) measurement of malignant lesion. T2w sections demonstrating the height of measurements for T2M sequence. Region of interest (ROI) measurements were drawn with the polygonal measurement tool an apical (A), midbase (B) and base (C). Suspicious lesions were additionally measured on the slice with its largest diameter (D dotted line). This image represents a confirmed prostate cancer of the left peripheral zone (arrowhead)
Prostate Biopsy Performance
All patients included in our study underwent a systematic prostate biopsy. Before the biopsy, rectal swabs and/or urine cultures were performed if clinically indicated. A periprostatic local anesthesia was injected under ultrasound-guidance. We took 12 cores, 6 from each prostate lobe, with a length of 15–22 mm. If a targeted fusion biopsy was performed in addition, 2 cores were taken from each suspicious lesion (defined as PIRADS ≥ 3).
Targeted biopsy was performed with a high-end ultrasound-machine (HiVison, Hitachi Medical Systems, Tokyo, Japan) [17,18,19,20].
Interpretation of Biopsy
Imaging abnormalities were defined as confirmed malignancies based on the interpretation of the biopsy results for the corresponding prostate regions. Additionally, the regions of the corresponding lesions were noted and compared with the imaging findings.
Data Collection
Measurements were performed by an independent assessor trained in the use of PACs measurement tools on a GE Workstation (Universal Viewer, GE, Boston).
For each MRI study, three regions of interest were drawn at representative axial sections: at the level of apex (pTa), mid-base (pTm) and base (pTb). Each ROI was drawn in three different regions per slice, including the right peripheral zone, left peripheral zone and transitional zone. Thus, a minimum of 9 ROIs were drawn per subject.
A differentiation was made between tumor-free (pT) and tumor-containing tissue (pCA). Tumor-containing tissue was defined as PIRADS ≥ 3 and Gleason Score ≠ 0. Prostate tissue without suspicious lesions (as determined by mpMRI and biopsy report) was measured bilaterally in the peripheral zone, unless the zone was completely affected such that a representative ROI could not be set. In this case, only malignant lesion measurements were recorded for this region. The slice with the largest tumor extent was also included to mark a representative ROI for the tumor-tissue (Fig. 3). Image measurements were performed in a structured manner suitable to inexperienced readers.
MRIs were originally marked in the T2w reconstruction of the T2M sequence. All corresponding measurements were mapped to other sequences by table position and synchronization of image stacks. If the automatic matching failed, corrections to the mapping between sequences could be made manually. ROIs were always copied from the first sequence measured to all other corresponding sequences. Therefore, identical ROI shapes and sizes were ensured to match measurements between sequences as closely as possible. Mean and minimum transverse relaxation time (T2) values in each ROI were recorded.
Measures of Data Validity
To ensure data quality, incomplete data, studies of poor image quality and inconsistent data was rigorously sorted out.
In addition, the ROIs were marked by an independent reader. ROIs were drawn leaving a margin to the edge of each structure to avoid including other tissue. ROIs were made as large as possible to get a representative average value per region. ROIs were not placed in areas containing artifacts.
All measurements and the markings of the ROIs were reviewed by an expert reader (AMB) with 6 years of experience in reading prostate MRI. ROIs were however only redrawn if the desired prostate region was not accurately included, in order to test the suitability of the approach for an inexperienced reader.
Statistical Tests
Statistical analysis was conducted using commercially available statistical software, including SPSS, version 21.0 (© 1989–2012, IBM, Armonk, NY, USA; MedCalc Software bv, Ostend, Belgium; RStudio, PBC, Boston, MA, USA). The normal distribution was determined using the Shapiro-Wilk test. Categorical variables are presented as percentages, continuous variables as mean ± standard deviation or median and interquartile ranges if the distribution is not normal. The non-parametric were assessed using the Mann-Whitney-U-test. To determine any correlation, the Spearman test was performed. A receiver operating characteristic (ROC) was used to identify the cut-off value that achieved the best balance between sensitivity and specificity. Using a linear mixed-effects model (LMM) fit by Restricted Maximum Likelihood (REML), we assessed the differences in transverse relaxation times (T2) between cancerous and healthy prostate tissues. The model included fixed effects for tissue type (cancerous vs. healthy) and random intercepts for subjects to account for inter-individual variability. P-values less than 0.05 were considered statistically significant.
Results
Patient Population
We included all patients between 08/2018 and 07/2019, who received a prostate MRI to rule out a pCA in our institute. Of these 260 patients, in 91 cases T2M were part of the image protocol. Of these 91 remaining patients, 61 received a prostate-biopsy. After excluding patients based on image quality criteria, as assessed by a radiologist with 6 years of experience, a total of 54 patients were included (Fig. 1).
Our patient cohort thus contained 37 patients with biopsy confirmed pCA (pCAP) and 17 patients without a pCA (pCAN). Mean age was 65.5 years (± 7.7 years, min: 42 years, max 83 years). Patient age was comparable between groups (p = 0.6915; Table 1). Mean PSA level of the pCAP group was 36,1 ng/ml (95% CI: 10.7–61.5 ng/ml).
Biopsy Results
The biopsy results confirmed pCA in 37 cases. The most common Gleason score was 7. The mean interval between MRI scan and prostate biopsy was 21 days (95% CI: 14–28 days). An example of patients with pCA detected on our MRI protocol is included in Fig. 3.
mpMRI Results
The PIRADS value was previously determined by radiologists. PIRADS was looked at per lesion in case of several lesions. The average PIRADS was 3.9 (± 1.03), the most frequent PIRADS score was 5 (n = 21; Table 3). T2M values in peripheral zones of healthy tissue but also in diseased tissue were not normally distributed. An overview of the T2M values of the peripheral zone is shown in Table 5.
The mean relaxation time for healthy tissue was significantly higher at 151.7 ms (95% CI: 146.9–156.5 ms) compared to malignant lesions, which averaged 73.5 ms (95% CI: 67.9–79.1 ms, p < 0.0001; Fig. 4). 95% of the pCA values were below 121.4 ms.
T2M in different peripheral regions of the prostate did not differ significantly (Table 4, Fig. 5).
Box-and-whisker plots of T2 mapping measurements per prostate region. T2-Mapping values (ms) are shown grouped by anatomic prostate region of all non malignant tissue measurements. Although there are slight differences in the average relaxation times of prostate tissue per prostate region, there is no significant overlap with malignant lesions measured across all prostate regions. The measurements of peripheral zone regions with cancer affection have been pooled into one group (pCA) for comparison. To represent the reader's differentiation task of comparing benign lesions of any given prostate zone to the average values of malignant lesions, we chose to pool pCA values. Table 5 provides a more detailed comparison of average relaxation times per region for both groups
The linear mixed-effect model showed that the relaxation time of the mean values in lesions is on average 82.7 ms less than the relaxation time of healthy tissue.
The Spearman test did not reveal any correlation or significant differences between the relaxation time and Gleason score in the diseased tissue. However, peripheral zone T2M values exhibited a correlation with PI-RADS score (p = 0.0194).
The ROC analysis of T2M-values resulted in an AUC of 0.973 (95% CI [0.951–0.987], p < 0.0001), with a threshold value of ≤ 109.2 ms, (sensitivity: 94.07%, specificity: 92.06%) for the differentiating between pT and pCA (Figs. 6 and 7). We analyzed the relationship of T2M-values and the classification into pT and pCA using logistic regression. The logistic regression model was found to be statistically significant (χ2 = 274,2, p-value < 0.0001). The model explained 78,8% (Nagelkerke R2) of the variance in cancer presence and correctly classified 93% of cases. The odds ratio for T2M-values was 0,89 (95% CI 0.87 to 0.92), with a coefficient of -0.110 (Standard error: 0.013; p < 0.001; Fig. 8).
Discussion
One of the challenges radiologists face in differentiating between benign and malignant tissue of the prostate is that conventional T2w and DWI sequences provide qualitative rather than quantitative imaging information. Intra-individual comparisons have shown large heterogeneity in quantitative measurements for these sequences when comparing quantitative measurements in [21]. Diagnosis of prostate cancer remains a subjective process and relies primarily on experienced radiologists [22, 23]. However, our study demonstrates that using quantitative T2M values can aid in distinguishing between healthy tissue and tumors in the peripheral zone of the prostate. The quantitative values for pCA were significantly lower than those for healthy tissue (73.5 ms vs. 151.7 ms). Through ROC analysis, a threshold of 109.2 ms was statistically determined.
Using biopsy confirmation as a reference method, detection of malignant prostate tissue based on quantitative measurements derived from high-resolution T2M sequences can lead to good differentiation of lesions in the peripheral zone.
There was no significant correlation between Gleason Score and tissue measurement. In our study, therefore, the aggressiveness of the lesion cannot be inferred from the measured value.
Our measured T2M values are consistent with those reported in previous studies affirming the reliability of our measurement techniques [3, 7, 24, 25]. Our proposed threshold lies between the two thresholds identified by Yamauchi et al. (99 ms) and Mai et al. (134 ms) [3, 24]. However, our threshold showed a higher sensitivity in our cohort than those two mentioned above. Liu et al. determined reference values in their study on T2M (100 +– 10 ms for malignant lesions, 149 +–32 ms for healthy tissue) [26]. Although our values differ slightly, they both show that the values for malignant lesions are significantly lower than for healthy tissue. Our proposed threshold for the best possible sensitivity and specificity was 109.2 ms, which is close to the values of Liu et al.
As demonstrated in previous studies, a quantitative T2M sequence can provide a repeatable, measurable value for diagnosing malignant-suspicious prostate findings [3, 7, 25]. By utilizing the measured values of the T2M, it is possible to differentiate between benign and cancer-related tissue. This method enabled sufficient differentiation of malignant findings to be shown, particularly in the peripheral zone. Wu et al. showed in their study on 31 patients that the specificity and sensitivity are higher if the T2M values are also used additionally to the normal T2w values [27]. Potential uses of quantification in the T2M sequence could be both assessment of the prostate in the case of elevated laboratory parameters (prostate-specific antigen) and follow-up monitoring after therapy to see the response. This is also relevant if the prostate is palpable or if the prostate carcinoma has been histologically confirmed. T2M could help to evaluate the spread of the carcinoma prior to surgery. T2M could thus increase the value of a contrast agent-free mpMRI. In addition, the T2M is also helpful for inexperienced readers since malignant findings appear in a single sequence and do not have to be linked over several sequences. Inexperienced readers usually must assess and compare different sequences of the MRI in order to assess the presence of tumor-free or tumor-containing tissue. The T2M sequence and the significant threshold identified in this study could help readers to assess the occurrence of malignant tissue more reliably by using only one sequence. The suspicion of an assumed malignant change could be confirmed based on measured values. Accelerated T2M could be a feasible addition to standardized multi-parametric MRI of the prostate and could further aid automated lesion detection with quantitative measurements. While we used a simple thresholding method the addition of fingerprinting and pattern recognition approaches could help with characterization of lesions that are more difficult to evaluate, such as transitional zone lesions [28]. In breast cancer diagnostics, for example, studies have already shown that the T2M value of malignant and benign zones in the breast differs [26]. According to Mai et al. the synthetic T2w contrast calculated from the T2M is equal to anatomy and diagnostic accuracy compared to conventional T2w [3]. Therefore, there could be the possibility to use only one sequence (T2M) and its synthetic T2w for detection of prostate lesions, replacing the standard T2w acquisition.
This study has some limitations that must be considered when interpreting the results. First, there was not much data acquired with the novel T2M sequence in the clinical database when the study was executed. In some of them, no pathological examination was carried out, so that the results could not be correlated with pathological data. As a result, the number of patients examined is rather small. However, compared to other studies, our study did not examine fewer patients [7, 24, 25].
Furthermore, the exact correlation of the pathology with the image values is only possible to a limited extent. Although the pathological finding confirms e.g. the presence of a carcinoma in a certain zone, this does not mean that the entire zone is affected. It was therefore still necessary for the reader to independently examine the image for the lesion, find it and mark it. Inaccuracies can happen here. To prevent this, different sequences were compared. In particular, the ADC and DWI sequences were helpful in pinpointing the actual lesion. Another limitation is that not all patients had the same type of pathological examination. Prostatectomy is certainly the most accurate. However, this type of histopathology was only performed in a few patients.
Finally, there is the inaccuracy of the measurement of healthy tissue. Here, the examiner chose ROIs in the areas that were designated as non-malignant by the pathology. It cannot be ruled out that in those areas are signal changes too, e.g. because of benign prostatitis, which also leads to signal changes. These benign lesions were not investigated further in our study, in contrast to the study by Hepp et al. [7]. The investigators tried to prevent this by setting the ROIs in healthy tissue as large as possible, paying attention to homogeneity. This minimizes distortion of the results due to possible small areas with changes. There was no significant correlation between Gleason Score and tissue measurement. In our study, therefore, the aggressiveness of the lesion cannot be inferred from the measured value.
Conclusion
Our investigation confirmed that there are significant differences in the T2M sequence between the relaxation time in healthy prostate tissue and prostate carcinomas in the peripheral zone. We recommend a threshold of 109.2 ms. Our investigation did not show any correlation between the measured value and Gleason Score.Therefore, in the future, the quantitative determination in the T2M sequence could help radiologists to better assess suspicious lesions of the prostate.
Data Availability
All MR images in this study are owned by University Hospital Frankfurt, Frankfurt, Germany, for research purposes and cannot be made publicly available owing to patient privacy and ethical concerns.
References
Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877-892. https://doi.org/10.1016/j.euo.2021.09.006
Nguyen-Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med. 2016;46(6):484-490. https://doi.org/10.1053/j.semnuclmed.2016.07.002
Mai J, Abubrig M, Lehmann T, et al. T2 mapping in prostate cancer. Invest Radiol. 2019;54(3):146-152. https://doi.org/10.1097/RLI.0000000000000520
Grivas N, Lardas M, Espinós EL, et al. Prostate Cancer Detection Percentages of Repeat Biopsy in Patients with Positive Multiparametric Magnetic Resonance Imaging (Prostate Imaging Reporting and Data System/Likert 3-5) and Negative Initial Biopsy. A Mini Systematic Review. Eur Urol. 2022;82(5):452-457. https://doi.org/10.1016/j.eururo.2022.07.025
Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 21: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–351. https://doi.org/10.1016/j.eururo.2019.02.033
Klingebiel M, Schimmöller L, Weiland E, et al. Value of T2 mapping MRI for prostate cancer detection and classification. J Magn Reson Imaging. 2022;56(2):413-422. https://doi.org/10.1002/jmri.28061
Hepp T, Kalmbach L, Kolb M, et al. T2 mapping for the characterization of prostate lesions. World J Urol. 2022;40(6):1455-1461. https://doi.org/10.1007/s00345-022-03991-8
Hoang Dinh A, Souchon R, Melodelima C, et al. Characterization of prostate cancer using T2 mapping at 3T: a multi-scanner study. Diagn Interv Imaging. 2015;96(4):365-372. https://doi.org/10.1016/j.diii.2014.11.016
Hilbert T, Sumpf TJ, Weiland E, et al. Accelerated T2 mapping combining parallel MRI and model-based reconstruction: GRAPPATINI. J Magn Reson Imaging. 2018;48(2):359-368. https://doi.org/10.1002/jmri.25972
Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210. https://doi.org/10.1002/mrm.10171
Roux M, Hilbert T, Hussami M, Becce F, Kober T, Omoumi P. MRI T2 Mapping of the Knee Providing Synthetic Morphologic Images: Comparison to Conventional Turbo Spin-Echo MRI. Radiology. 2019;293(3):620-630. https://doi.org/10.1148/radiol.2019182843
Gruenebach N, Abello Mercado MA, Grauhan NF, et al. Clinical feasibility and validation of the accelerated T2 mapping sequence GRAPPATINI in brain imaging. Heliyon. 2023;9(4):e15064. https://doi.org/10.1016/j.heliyon.2023.e15064
Raudner M, Schreiner MM, Hilbert T, et al. Clinical implementation of accelerated T2 mapping: Quantitative magnetic resonance imaging as a biomarker for annular tear and lumbar disc herniation. Eur Radiol. 2021;31(6):3590-3599. https://doi.org/10.1007/s00330-020-07538-6
Vietti Violi N, Hilbert T, Bastiaansen JAM, et al. Patient respiratory-triggered quantitative T2 mapping in the pancreas. J Magn Reson Imaging. 2019;50(2):410-416. https://doi.org/10.1002/jmri.26612
Li S, Liu J, Zhang F, et al. Novel T2 mapping for evaluating cervical cancer features by providing quantitative T2 maps and synthetic morphologic images: A preliminary study. J Magn Reson Imaging. 2020;52(6):1859-1869. https://doi.org/10.1002/jmri.27297
Draveny R, Ambarki K, Han F, et al. Comparison of T2 Quantification Strategies in the Abdominal-Pelvic Region for Clinical Use. Invest Radiol. 2022;57(6):412-421. https://doi.org/10.1097/RLI.0000000000000852
Wenzel M, von Hardenberg J, Welte MN, et al. Monoprophylaxis With Cephalosporins for Transrectal Prostate Biopsy After the Fluoroquinolone-Era: A Multi-Institutional Comparison of Severe Infectious Complications. Front Oncol. 2021;11:684144. https://doi.org/10.3389/fonc.2021.684144
Wenzel M, Theissen L, Preisser F, et al. Complication Rates After TRUS Guided Transrectal Systematic and MRI-Targeted Prostate Biopsies in a High-Risk Region for Antibiotic Resistances. Front Surg. 2020;7:7. https://doi.org/10.3389/fsurg.2020.00007
Wenzel M, Welte MN, Theissen LH, et al. Comparison of Complication Rates with Antibiotic Prophylaxis with Cefpodoxime Versus Fluoroquinolones After Transrectal Prostate Biopsy. Eur Urol Focus. 2021;7(5):980-986. https://doi.org/10.1016/j.euf.2020.11.006
Wenzel M, Preisser F, Wittler C, et al. Correlation of MRI-Lesion Targeted Biopsy vs. Systematic Biopsy Gleason Score with Final Pathological Gleason Score after Radical Prostatectomy. Diagnostics (Basel). 2021;11(5). https://doi.org/10.3390/diagnostics11050882
Scherer J, Nolden M, Kleesiek J, et al. Joint imaging platform for federated clinical data analytics. JCO Clin Cancer Inform. 2020;4:1027-1038. https://doi.org/10.1200/CCI.20.00045
Ruprecht O, Weisser P, Bodelle B, Ackermann H, Vogl TJ. MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol. 2012;81(3):456-460. https://doi.org/10.1016/j.ejrad.2010.12.076
Garcia-Reyes K, Passoni NM, Palmeri ML, et al. Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging. 2015;40(1):134-142. https://doi.org/10.1007/s00261-014-0197-7
Yamauchi FI, Penzkofer T, Fedorov A, et al. Prostate cancer discrimination in the peripheral zone with a reduced field-of-view T(2)-mapping MRI sequence. Magn Reson Imaging. 2015;33(5):525-530. https://doi.org/10.1016/j.mri.2015.02.006
Liu W, Turkbey B, Sénégas J, et al. Accelerated T2 mapping for characterization of prostate cancer. Magn Reson Med. 2011;65(5):1400-1406. https://doi.org/10.1002/mrm.22874
Liu L, Yin B, Shek K, et al. Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J Int Med Res. 2018;46(5):1928-1935. https://doi.org/10.1177/0300060517721071
Wu L-M, Yao Q-Y, Zhu J, et al. T2* mapping combined with conventional T2-weighted image for prostate cancer detection at 30T MRI: a multi-observer study. Acta Radiol. 2017;58(1):114–120. https://doi.org/10.1177/0284185116633916
Panda A, Obmann VC, Lo W-C, et al. MR fingerprinting and ADC mapping for characterization of lesions in the transition zone of the prostate gland. Radiology. 2019;292(3):685-694. https://doi.org/10.1148/radiol.2019181705
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by "NUM 2.0" (FKZ: 01KX2121), the REACT-EU project "KITE" (grant number: EFRE-0801977, Plattform für KI-Translation Essen, https://kite.ikim.nrw/), FWF "enFaced 2.0" (grant number: KLI-1044, https://enfaced2.ikim.nrw/).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Andreas Bucher, Alicia Polk, Alica Koehler, Mike Wenzel and Julia Dietz. The first draft of the manuscript was written by Andreas Bucher and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
For this IRB approved (No. 19-299), retrospective case-control study, the hospital's PACS was searched by an independent investigator for prostate MRIs between 08/2018 and 07/2019.
Consent to Participate
For the retrospective case-control study, no consent to participate was needed.
Consent to Publish
For the anonymized data, no consent to publish is needed.
Competing Interests
R.S. and T.H. are employed by Siemens Healthineers. T.P. receives funding from Berlin Institute of Health (Advanced Clinician Scientist Grant, Platform Grant), Ministry of Education and Research (BMBF, 01KX2021 (RACOON), 01KX2121 („NUM 2.0“, RACOON), 68GX21001A, 01ZZ2315D), German Research Foundation (DFG, SFB 1340/2), European Union (H2020, CHAIMELEON: 952172, DIGITAL, EUCAIM:101100633) and reports research agreements (no personal payments, outside of submitted work) with AGO, Aprea AB, ARCAGY-GINECO, Astellas Pharma Global Inc. (APGD), Astra Zeneca, Clovis Oncology, Inc., Holaira, Incyte Corporation, Karyopharm, Lion Biotechnologies, Inc., MedImmune, Merck Sharp & Dohme Corp, Millennium Pharmaceuticals, Inc., Morphotec Inc., NovoCure Ltd., PharmaMar S.A. and PharmaMar USA, Inc., Roche, Siemens Healthineers, and TESARO Inc., and fees for a book translation (Elsevier B.V.). A.M.B.: Bayer, Guebert, Siemens Healthineers (Consulting fees and travel Support).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Short Synopsis
In this investigation, we found a good correlation between quantitative T2 mapping measurements of the prostate in 36 prostate cancer patients and biopsy results. Malignant lesions exhibited a significant reduction in T2 relaxation time (151.7 ms [95% CI: 160.4–143.6 ms] vs. 80.9 ms [95% CI: 78.2–60.4 ms]). The linear mixed-effects model, fitted by REML (R Statistics), revealed a difference of -82.7 ms and -42.8 ms for average and minimum values, respectively, between cancerous and healthy tissue.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bucher, A.M., Egger, J., Dietz, J. et al. Value of MRI - T2 Mapping to Differentiate Clinically Significant Prostate Cancer. J Digit Imaging. Inform. med. (2024). https://doi.org/10.1007/s10278-024-01150-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10278-024-01150-6