Abstract
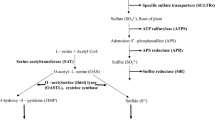
Cysteine biosynthesis is directed by the successive commitments of serine acetyltransferase, and O-acetylserine (thiol) lyase (OASTL) compounds, which subsequently frame the decameric cysteine synthase complex. The isoforms of OASTL are found in three compartments of the cell: the cytosol, plastid, and mitochondria. In this investigation, we first isolated putative chloroplastic OASTL (Ch-OASTL) from Leucaena leucocephala, and the Ch-OASTL was then expressed in BL21-competent Escherichia coli. The putative Ch-OASTL cDNA clone had 1,543 base pairs with 391 amino acids in its open reading frame and a molecular weight of 41.54 kDa. The purified protein product exhibited cysteine synthesis ability, but not mimosine synthesis activity. However, they both make the common α-aminoacrylate intermediate in their first half reaction scheme with the conventional substrate O-acetyl serine (OAS). Hence, we considered putative Ch-OASTL a cysteine-specific enzyme. Kinetic studies demonstrated that the optimum pH for cysteine synthesis was 7.0, and the optimum temperature was 40 °C. In the cysteine synthesis assay, the Km and kcat values were 838 ± 26 µM and 72.83 s−1 for OAS, respectively, and 60 ± 2 µM and 2.43 s−1 for Na2S, respectively. We can infer that putative Ch-OASTL regulatory role is considered a sensor for sulfur constraint conditions, and it acts as a forerunner of various metabolic compound molecules.






Similar content being viewed by others
Abbreviations
- Acetyl-CoA:
-
Acetyl coenzymeA
- AI:
-
Aliphatic index
- AMBER:
-
Assisted model building with energy refinement
- BSAS:
-
β-substituted alanine synthase
- CAS:
-
β-cyanoalanine synthase
- Ch-OASTL:
-
Chloroplastic OASTL
- CSC:
-
Cysteine synthase complex
- DMP:
-
2,5-dimethyl-3-pyridinol
- FMO:
-
Fragment molecular orbital
- GRAVY:
-
Grand average of hydropathicity
- 3H4P:
-
3-hydroxy-4-pyridone
- IFIE:
-
Inter-fragment interaction energy
- II:
-
Instability index
- M9:
-
Minimal nutrient agar
- NPAA:
-
Nonprotein amino acid
- OAS:
-
O-acetyl serine
- OASTL:
-
O-acetylserine (thiol) lyase
- PAICS:
-
Parallelized ab initio calculation system
- PDB:
-
Protein data bank
- pI:
-
Theoretical isoelectric point
- SAT:
-
Serine acetyltransferase
References
Álvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with l-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Berkowitz O, Wirtz M, Wolf A, Kuhlmann J, Hell R (2002) Use of biomolecular interaction analysis to elucidate the regulatory mechanism of the cysteine synthase complex from Arabidopsis thaliana. J Biol Chem 277:30629–30634
Bermúdez MA, Páez-Ochoa MA, Gotor C, Romero LC (2010) Arabidopsis S-Sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control. Plant Cell 22:403–416
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Ann Rev Cell Develop Biol 16:1–18
Blumenthal SG, Hendrickson HR, Abrol YP, Conn EE (1968) Cyanide metabolism in higher plants. 3. The biosynthesis of beta-cyanolanine. J Biol Chem 243:5302–5307
Bonner ER, Cahoon RE, Knapke SM, Jez JM (2005) Molecular basis of cysteine biosynthesis in plants: structural and functional analysis of O-acetylserine sulfhydrylase from Arabidopsis thaliana. J Biol Chem 280:38803–38813
Burkhard P, Rao GS, Hohenester E, Schnackerz KD, Cook PF, Jansonius JN (1998) Three-dimensional structure of O-acetylserine sulfhydrylase from Salmonella typhimurium. J Mol Biol 283:121–133
Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323:95–101
Dharavath S, Raj I, Gourinath S (2017) Structure-based mutational studies of O-acetylserine sulfhydrylase reveal the reason for the loss of cysteine synthase complex formation in Brucella abortus. Biochem J 474:1221–1239
Dixon DP, Davis BG, Edwards R (2002) Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J Biol Chem 277:30859–30869
Droux M (2004) Sulfur assimilation and the role of sulfur in plant metabolism: a survey. Photosyn Res 79:331–348
Droux M, Ruffet ML, Douce R, Job D (1998) Interactions between serine acetyltransferase and O-acetylserine (thiol) lyase in higher plants-structural and kinetic properties of the free and bound enzymes. Eur J Biochem 255:235–245
Eliot AC, Kirsch JF (2004) Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Ann Rev Biochem 73:383–415
Feldman-Salit A, Wirtz M, Hell R, Wade RC (2009) A mechanistic model of the cysteine synthase complex. J Mol Biol 386:37–59
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Francois JA, Kumaran S, Jez JM (2006) Structural basis for interaction of O-acetylserine sulfhydrylase and serine acetyltransferase in the Arabidopsis cysteine synthase complex. Plant Cell 18:3647–3655
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Garcia I, Castellano JM, Vioque B, Solano R, Gotor C, Romero LC (2010) Mitochondrial beta-cyanoalanine synthase is essential for root hair formation in Arabidopsis thaliana. Plant Cell 22:3268–3279
Gerwick BC, Ku SB, Black CC (1980) Initiation of sulfate activation: a variation in C4 photosynthesis plants. Science 209:513–515
Gotor C, Romero LC (2013) S-sulfocysteine synthase function in sensing chloroplast redox status. Plant Signal Behav 8:e23313
Goudey JS, Tittle FL, Spencer MS (1989) A role for ethylene in the metabolism of cyanide by higher plants. Plant Physiol 89:1306–1310
Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K (2000) β-Cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis. Plant Physiol 123:1163–1171
Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R (2008) Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 20:168–185
Hell R, Bergmann L (1990) λ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta 180:603
Hell R, Wirtz M (2008) Metabolism of cysteine in plants and phototrophic bacteria. In: Hell R, Dahl C, Knaff D, Leustek T (eds) Sulfur metabolism in phototrophic organisms. Springer, Dordrecht, pp 59–91
Hesse H, Lipke J, Altmann T, Hofgen R (1999) Molecular cloning and expression analyses of mitochondrial and plastidic isoforms of cysteine synthase (O-acetylserine(thiol)lyase) from Arabidopsis thaliana. Amino Acids 16:113–131
Howarth JR, Domínguez-Solís JR, Gutiérrez-Alcalá G, Wray JL, Romero LC, Gotor C (2003) The serine acetyltransferase gene family in Arabidopsis thaliana and the regulation of its expression by cadmium. Plant Mol Biol 51:589–598
Ishikawa T, Ishikura T, Kuwata K (2009) Theoretical study of the prion protein based on the fragment molecular orbital method. J Comput Chem 30:2594–2601
Kitaura K, Ikeo E, Asada T, Nakano T, Uebayasi M (1999a) Fragment molecular orbital method: an approximate computational method for large molecules. Chem Phys Lett 313:701–706
Kitaura K, Sawai T, Asada T, Nakano T, Uebayasi M (1999b) Pair interaction molecular orbital method: an approximate computational method for molecular interactions. Chem Phys Lett 312:319–324
Krueger S, Niehl A, Lopez Martin MC, Steinhauser D, Donath A, Hildebrandt T, Romero LC, Hoefgen R, Gotor C, Hesse H (2009) Analysis of cytosolic and plastidic serine acetyltransferase mutants and subcellular metabolite distributions suggests interplay of the cellular compartments for cysteine biosynthesis in Arabidopsis. Plant Cell Environ 32:349–367
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kumaran S, Jez JM (2007) Thermodynamics of the interaction between O-acetylserine sulfhydrylase and the C-terminus of serine acetyltransferase. Biochemistry 46:5586–5594
Kuske CR, Ticknor LO, Guzman E, Gurley LR, Valdez JG, Thompson ME, Jackson PJ (1994) Purification and characterization of O-acetylserine sulfhydrylase isoenzymes from Datura innoxia. J Biol Chem 269:6223–6232
Lunn JE, Droux M, Martin J, Douce R (1990) Localization of ATP sulfurylase and O-acetylserine(thiol)lyase in Spinach leaves. Plant Physiol 94:1345–1352
Mendoza-Cozatl DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Meyer AJ (2008) The integration of glutathione homeostasis and redox signaling. J Plant Physiol 165:1390–1403
Meyer AJ, Hell R (2005) Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res 86:435–457
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Mino K, Yamanoue T, Sakiyama T, Eisaki N, Matsuyama A, Nakanishi K (1999) Purification and characterization of serine acetyltransferase from Escherichia coli partially truncated at the C-terminal region. Biosci Biotechnol Biochem 63:168–179
Mino K, Hiraoka K, Imamura K, Sakiyama T, Eisaki N, Matsuyama A, Nakanishi K (2000) Characteristics of serine acetyltransferase from Escherichia coli deleting different lengths of amino acid residues from the C-terminus. Biosci Biotechnol Biochem 64:1874–1880
Ng BH, Anderson JW (1978) Chloroplast cysteine synthases of Trifolium repens and Pisum sativum. Phytochemistry 17:879–885
Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. Arabidopsis Book Am Soc Plant Biol 9:e0142
Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer AJ (2008) Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J 53:999–1012
Picciocchi A, Douce R, Alban C (2003) The plant biotin synthase reaction. Identification and characterization of essential mitochondrial accessory protein components. J Biol Chem 278:24966–24975
Pilon-Smits EA, Garifullina GF, Abdel-Ghany S, Kato S, Mihara H, Hale KL, Burkhead JL, Esaki N, Kurihara T, Pilon M (2002) Characterization of a NifS-like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiol 130:1309–1318
Ponder JW, Case DA (2003) Force fields for protein simulations. Adv Protein Chem 66:27–85
Rashid MHU, Iwasaki H, Parveen S, Oogai S, Fukuta M, Hossain MA, Anai T, Oku H (2018a) Cytosolic cysteine synthase switch cysteine and mimosine production in Leucaena leucocephala. Appl Biochem Biotechnol 186:613–632
Rashid MHU, Iwasaki H, Oogai S, Fukuta M, Parveen S, Hossain MA, Anai T, Oku H (2018b) Molecular characterization of cytosolic cysteine synthase in Mimosa pudica. J Plant Res 131:319–329
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509
Rea PA (2012) Phytochelatin synthase: of a protease a peptide polymerase made. Physiol Plant 145:154–164
Richau KH, Kaschani F, Verdoes M, Pansuriya TC, Niessen S, Stüber K, Colby T, Overkleeft HS, Bogyo M, Van der Hoorn RAL (2012) Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol 158:1583–1599
Romero LC, Aroca MÁ, Laureano-Marín AM, Moreno I, García I, Gotor C (2014) Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant 7:264–276
Ruffet ML, Droux M, Douce R (1994) Purification and kinetic properties of serine acetyltransferase free of O-acetylserine(thiol)lyase from spinach chloroplasts. Plant Physiol 104:597–604
Saito K (2004) Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol 136:2443–2450
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schmidt A (1986) Regulation of sulfur metabolism in plants. In: Behnke HD, Esser K, Kubitzki K, Runge M, Ziegler H (eds) Progress in botany. Progress in botany/fortschritte der botanik, vol 48. Springer, Berlin, Heidelberg
Scharer MA, Eliot AC, Grutter MG, Capitani G (2011) Structural basis for reduced activity of 1-aminocyclopropane-1-carboxylate synthase affected by a mutation linked to andromonoecy. FEBS Lett 585:111–114
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Ann Rev Plant Biol 62:157–184
Van Hoewyk D, Pilon M, Pilon-Smits EAH (2008) The functions of NifS-like proteins in plant sulfur and selenium metabolism. Plant Sci 174:117–123
Wachter A, Wolf S, Steininger H, Bogs J, Rausch T (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41:15–30
Warrilow AG, Hawkesford MJ (2000) Cysteine synthase (O-acetylserine (thiol) lyase) substrate specificities classify the mitochondrial isoform as a cyanoalanine synthase. J Exp Bot 51:985–993
Wirtz M, Droux M (2005) Synthesis of the sulfur amino acids: cysteine and methionine. Photosynth Res 86:345–362
Wirtz M, Hell R (2006) Functional analysis of the cysteine synthase protein complex from plants: structural, biochemical and regulatory properties. J Plant Physiol 163:273–286
Wirtz M, Droux M, Hell R (2004) O-acetylserine (thiol) lyase: an enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J Exp Bot 55:1785–1798
Wirtz M, Birke H, Heeg C, Müller C, Hosp F, Throm C, König S, Feldman-Salit A, Rippe K, Petersen G, Wade RC, Rybin V, Scheffzek K, Hell R (2010) Structure and function of the hetero-oligomeric cysteine synthase complex in plants. J Biol Chem 285:32810–32817
Wirtz M, Beard KFM, Lee CP, Boltz A, Schwarzlaender M, Fuchs C, Meyer AJ, Heeg C, Sweetlove LJ, Ratcliffe RG, Hell R (2012) Mitochondrial cysteine synthase complex regulates O-acetylserine biosynthesis in plants. J Biol Chem 287:27941–27947
Yafuso JT, Negi VS, Bingham JP, Borthakur D (2014) An O-acetylserine (thiol) lyase from Leucaena leucocephala is a cysteine synthase but not a mimosine synthase. Appl Biochem Biotechnol 173:1157–1168
Yamada T, Komoto J, Takata Y, Ogawa H, Pitot HC, Takusagawa F (2003) Crystal structure of serine dehydratase from rat liver. Biochemistry 42:12854–12865
Yi H, Juergens M, Jez JM (2012) Structure of soybean β-Cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. Plant Cell 24:2696–2706
Yip WK, Yang SF (1988) Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol 88:473–476
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins evolving genes and proteins. Academic Press, Cambridge, pp 97–166
Acknowledgements
We are thankful to Dr. Shinichi Gima of the Instrumental Research Center, University of the Ryukyus and Dr. Michael Chandra Roy, Okinawa Institute of Science and Technology for giving specialized help amid the LC–MS/MS analyses. We sincerely thank to Dr. Rafiq Islam (Professor, Department of Natural Sciences, Northwest Missouri State University) for useful discussion and necessary corrections of our manuscript. The author also thank Dr. Takeshi Ishikawa, Nagasaki University Graduate School of Biomedical Sciences for providing us the PAICS program.
Author information
Authors and Affiliations
Contributions
MHR and SO equally contributed in this experiment. MHR performed all the experiment, data analysis and draft of the manuscript; SO performed the molecular dynamics simulation; SP performed the experiments and analyzed the data; FM supervised the student, developed the concepts and designed experiments, and edited the manuscript; MAH and HI supervised the student; MI guided MHR in the experiment and edited the manuscript, and HO supervised the student, performed the molecular dynamics simulation and analysis, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accession numbers
The information of protein sequences can be found in Gene bank under the following accessions: 5JJC (crystal structure of double mutant (Q96A-Y125A) O-acetyl serine sulfhydralase from Brucella abortus), 1z7w (the crystal structure of O-acetylserine sulfhydrylase of Arabidopsis thaliana), LC342269 (Ch-OASTL of Leucaena leucocephala), BAK38374 (cysteine synthase, Mimosa pudica), LC306827 (Cy-OASTL, Leucaena leucocephala), and AHG97874 (Cy-O-acetylserine thiol lyase, Leucaena leucocephala).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harun-Ur-Rashid, M., Oogai, S., Parveen, S. et al. Molecular cloning of putative chloroplastic cysteine synthase in Leucaena leucocephala. J Plant Res 133, 95–108 (2020). https://doi.org/10.1007/s10265-019-01158-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01158-y