Abstract
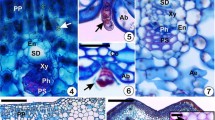
The expression of plant secondary metabolism is strongly controlled by plant both in time and space. Although the variation of secondary metabolites, such as soluble and structural phenolics (e.g., lignins), has been largely observed in gall-inducing insects, and compared to their non-galled host organs, only a few datasets recording such variation are available. Accordingly, the relative importance of spatiotemporal variability in phenolic contents, and the influence of gall developmental stages on the original composition of host organs are poorly discussed. To address this knowledge gap, we histochemically determined the sites of polyphenol and lignin accumulation, and the polyphenol contents in three developmental stages of two calophyid galls and their correspondent host organs. Current results indicate that the compartmentalization of phenolics and lignins on Schinus polygama (Cav.) Cabrera follows a similar pattern in the two-calophyid galls, accumulating in the outer (the external tissue layers) and in the inner tissue compartments (the cell layers in contact with the gall chamber). The non-accumulation in the median compartment (median parenchyma layers of gall wall with vascular bundles, where gall inducer feeds) is important for the inducer, because its mouth apparatus enter in contact with the cells of this compartment. Also, the concentration of phenolics has opposite dynamics, decreasing in leaf galls and increasing in stem galls, in temporal scale, i.e., from maturation toward senescence. The concentration of phenolics in non-galled host organs, and in both galls indicated the extended phenotype of Calophya rubra (Blanchard) and C. mammifex Burckhardt & Basset (Hemiptera: Sternorrhyncha: Psylloidea: Calophyidae) over the same host plant metabolic potentiality.





Similar content being viewed by others
References
Abrahamson WG, McCrea KD, Whitwell AJ, Vernieri LA (1991) The role of phenolics in goldenrod ball gall resistance and formation. Biochem Syst Ecol 19:615–622
Agudelo I, Wagner ML, Gurni AA, Ricco RA (2013) Dinámica de polifenoles y estudio anatomo-histoquímico en Schinus longifolius (Lindl.) Speg. (Anacardiaceae) en respuesta a la infección por Calophya mammifex (Hemiptera–Calophyidae). BLACPMA 12:162–175
Attard E (2013) A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Cent Eur J Biol 8:48–53
Bedetti CS, Bragança GP, Isaias RMS (2014a) Influence of auxin and phenolic accumulation on the patterns of cell differentiation in distinct gall morphotypes on Piptadenia gonoacantha (Fabaceae). Aust J Bot 65:411–420
Bedetti CS, Modolo LV, Isaias RMS (2014b) The role of phenolics in the control of auxin in galls of Piptadenia gonoacantha (Mart) MacBr (Fabaceae: Mimosoideae). Biochem Syst Ecol 55:53–59
Berenbaum M, Zangerl A (1999) Genetic variation in cytochrome P450-based resistance to plant allelochemicals and insecticides. In: Olff H, Brown VK, Drent RH (eds) Herbivores: between plants and predators. Blackwell, Cambridge, pp 55–84
Bishop DL (2002) Gene expression of a vacuolar peroxidase with stress-induced pathogenesis in wheat sheaths. Physiol Mol Plant Pathol 61:65–71
Borg-Olivier O, Monties B (1993) Lignin, suberin, phenolic acids and tyramine in the suberized, wound-induced potato periderm. Phytochemistry 32:601–606
Bragança GP, Oliveira DC, Isaias RMS (2017) Compartmentalization of metabolites and enzymatic mediation in nutritive cells of Cecidomyiidae galls on Piper arboreum Aubl. (Piperaceae). J Plant Stud 6:11–22
Burckhardt D, Basset Y (2000) The jumping plant-lice (Hemiptera, Psylloidea) associated with Schinus (Anacardiaceae): systematics, biogeography and host plant relationships. J Nat Hist 34:57–155
Carmona D, Lajeunesse MJ, Johnson MTJ (2011) Plant traits that predict resistance to herbivores. Funct Ecol 25:358–367
Cipollini DF, Redman AM (1999) Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. J Chem Ecol 25:271–281
Close D, McArthur C (2002) Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 99:166
Cornell HV (1983) The secondary chemistry and complex morphology of galls formed by the Cynipinae (Hymenoptera): why and how? Am Midl Nat 110:225–234
Dawra RK, Makkar HPS, Singh B (1988) Total phenolics, condensed tannins, and protein-precipitable phenolics in young and mature leaves of oak species. J Agric Food Chem 36:951–953
Detoni ML, Vasconcelos EG, Rust NM, Isaias RMS, Soares GLG (2011) Seasonal variation of phenolic content in galled and non-galled tissues of Calliandra brevipes Benth (Fabaceae: Mimosoidae). Acta Bot Bras 25:601–604
Dias GD, Ferreira BG, Moreira GRP, Isaias RMS (2013a) Developmental pathway from leaves to galls induced by a sap-feeding insect on Schinus polygamus (Cav.) Cabrera (Anacardiaceae). An Acad Bras Cienc 85:187–200
Dias GG, Moreira GRP, Ferreira BG, Isaias RMS (2013b) Why do the galls induced by Calophya duvauae Scott on Schinus polygamus (Cav.) Cabrera (Anacardiaceae) change color? Biochem Syst Ecol 48:111–122
Donaldson JR, Stevens MT, Barnhill HR, Lindroth RL (2006) Age-related shifts in leaf chemistry of clonal aspen (Populus tremuloides). J Chem Ecol 32:1415–1429
Ferreira BG, Álvarez R, Avritzer SC, Isaias RM (2016) Revisiting the histological patterns of storage tissues: beyond the limits of gall-inducing taxa. Botany 95:173–184
Ferreira BG, Falcioni R, Guedes LM, Avritzer SC, Antunes WC, Souza LA, Isaias RMS (2017) Preventing false negatives for histochemical detection of phenolics and lignins in PEG-embedded plant tissues. J Histochem Cytochem 65:1–12
Ferreira BG, Oliveira DC, Moreira ASFP, Faria AP, Guedes LM, França MGC, Álvarez R, Isaias RMS (2018) Antioxidant metabolism in galls due to the extended phenotypes of the associated organisms. PLoS One 13:e0205364
Formiga AT, Gonçalves SJMR, Soares GLG, Isaias RMS (2009) Relações entre o teor de fenóis totais e o ciclo das galhas de Cecidomyiidae em Aspidosperma spruceanum Müll. Arg. (Apocynaceae). Acta Bot Bras 23:93–99
Guedes LM, Aguilera N, Becerra J, Hernández V, Isaias RSM (2016) Leaf and stem galls of Schinus polygamus (Cav.) Cabr (Anacardiaceae): anatomical and chemical implications. Biochem Syst Ecol 69:266–273
Guedes LM, Aguilera N, Ferreira BG, Becerra J, Hernández V, Isaias RSM (2018a) Anatomical and phenological implications between Schinus polygama (Cav.) (Cabrera) (Anacardiaceae) and the galling insect Calophya rubra (Blanchard) (Hemiptera: Psylloidea). Plant Biol. 20:507–515
Guedes LM, Aguilera N, Ferreira BG, Becerra J, Sáez K, Pérez C, Isaias RMS (2018b) Factors influencing the morphogenesis of galls induced by Calophya mammifex (Calophyidae) on Schinus polygama (Anacardiaceae) leaves. Botany 96:589–599
Gulmon SL, Mooney HA (1986) Costs of defense and their effects on plant productivity. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 681–698
Harborne JB, Grayer RJ (2013) The anthocyanins. In: Harborne JB (ed) The flavonoids: advances in research since 1980. Chapman and Hall, London, pp 1–18
Hartley SE (1998) The chemical composition of plant galls: are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 113:492–501
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 3:283–335
Higuchi T (1985) Biosynthesis and biodegradation of wood components. Academic Press, New York
Hori K (1992) Insect secretion and their effect on plant growth, with special reference to hemipterans. In: Shorthouse JD, Rohfristsch O (eds) Biology of insect-induced galls. Oxford University Press, New York, pp 157–170
Isaias RSM, Oliveira DC (2014) Gall phenotypes-product of plant cells defensive responses to the inducers attack. In: Fernandes GW, Santos JC (eds) Neotropical insect galls. Springer, Dordrecht, pp 273–290
Isaias RMS, Oliveira DC, Moreira ASFP, Soares GLG, Carneiro RGS (2015) The imbalance of redox homeostasis in arthropod-induced plant galls: mechanisms of stress generation and dissipation. Biochim Biophys Acta 1850:1509–1517
Isaias RSM, Ferreira BG, Alvarenga DR, Barbosa LR, Salminen JP, Steinbauer MJ (2018) Functional compartmentalisation of nutrients and phenolics in the tissues of galls induced by Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) on Eucalyptus camaldulensis (Myrtaceae). Aust Entomol 57:238–246
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book, New York
Kause A, Ossipov V, Haukioja E, Lempa K, Hanhimäki S, Ossipova S (1999) Multiplicity of biochemical factors determining quality of growing birch leaves. Oecologia 120:102–112
Kolattukudy PE (2011) Polyesters in higher plants. In: Babel W, Steinbuchel A (eds) Advances in biochemical engineering/biotechnology, vol 71. Biopolyesters 1. Springer, Berlin, pp 1–49
Lattanzio V, Lattanzio VM, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Filippo I (ed) Phytochemistry: advances in research. Research Signpost, Thiruvananthapuram, pp 23–67
Lu F, Marita JM, Lapierre C, Jouanin L, Morreel K, Boerjan W, Ralph J (2010) Sequencing around 5-hydroxyconiferyl alcohol-derived units in caffeic acid O-methyltransferase-deficient poplar lignins. Plant Physiol 153:569–579
Mani MS (1964) Ecology of plant galls. Dr. W. Junk Publishers, The Hague
Meyer J, Maresquelle HJ (1983) Anatomie des galles. Schweizerbart Science Publishers, Stuttgart
Moore BD, Andrew RL, Külheim C, Foley WJ (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 201:733–750
Motta LB, Kraus JE, Salatino A, Salatino MLF (2005) Distribution of metabolites in galled and non-galled foliar tissues of Tibouchina pulchra. Biochem Syst Ecol 33:971–981
Moura JC, Bonine CA, Viana OFJ, Dornelas MC, Mazzafera P (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52:360–376
Neilson EH, Goodger JQ, Woodrow IE, Møller BL (2013) Plant chemical defense: at what cost? Trends Plant Sci 18:250–258
Neutelings G (2011) Lignin variability in plant cell walls: contribution of new models. Plant Sci 181:379–386
Nyman T, Julkunen-Tiitto R (2000) Manipulation of the phenolic chemistry of willow by gall-inducing sawflies. Proc Natl Acad Sci USA 97:13184–13187
O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Pty, Melbourne
Oliveira DC, Moreira ASFP, Isaias RMS, Martini V, Rezende UC (2017) Sink status and photosynthetic rate of the leaflet galls induced by Bystracoccus mataybae (Eriococcidae) on Matayba guianensis (Sapindaceae). Front Plant Sci 8:1249
Patrick JW (1988) Assimilate partitioning in relation to crop productivity. HortScience 23:33–40
Patten AM, Cardenas CL, Cochrane FC, Laskar DD, Bedgar DL, Davin LB, Lewis NG (2005) Reassessment of effects on lignification and vascular development in the irx4 Arabidopsis mutant. Phytochemistry 66:2092–2107
Patten AM, Jourdes M, Brown EE, Laborie MP, Davin LB, Lewis NG (2007) Reaction tissue formation and stem tensile modulus properties in wild-type and p-coumarate3-hydroxylase downregulated lines of alfalfa, Medicago sativa (Fabaceae). Am J Bot 94:912–925
Price PW, Fernandes GW, Waring GL (1987) Adaptive nature of insect galls. Environ Entomol 16:15–24
Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2013) InfoStat versión 2013. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba
Rodríguez R (2011) Anacardiaceae. In: Rodríguez R, Marticorena C (eds) Flora de Chile. Ediciones Universidad de Concepción, Concepción, pp 88–103
Rohfritsch O (1992) Patterns in gall development. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect-induced galls. Oxford University Press, Oxford, pp 60–86
Stone GN, Schonrögge K (2003) The adaptive significance of insect gall morphology. Trends Ecol Evol 18:512–522
Teixeira CT, Oliveira DCD, Kuster VC, Isaias RMS (2017) Immunocytochemical demonstration of cell wall components related to tissue compartments in the globoid galls induced by Clinodiplosis sp. (Cecidomyiidae) on Croton floribundus Spreng. (Euphorbiaceae). Botany 96:9–18
Tooker JF, Helms AM (2014) Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. J Chem Ecol 40:742–753
Van Cutsem E, Simonart G, Degand H, Faber AM, Morsomme P, Boutry M (2011) Gel-based and gel-free proteomic analysis of Nicotiana tabacum trichomes identifies proteins involved in secondary metabolism and in the (a)biotic stress response. Proteomics 11:440–454
Van Dam NM, Horn M, Mares M, Baldwin IT (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27:547–568
Wang Y, Chantreau M, Sibout R, Hawkins S (2013) Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4:220
Wink M (1997) Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv Bot Res 25:141–169
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Wink M (2013) Evolution of secondary metabolites in legumes (Fabaceae). S Afr J Bot 89:164–175
Zangerl AR, Rutledge CE (1996) The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. Am Nat 147:599–608
Acknowledgements
This work was supported by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) under Grant 63140050 (National PhD/2014-fellowship) awarded to LMG, Projects REDI170025 and MEC80170028 funded by CONICYT, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors are grateful to PD Dr. Daniel Burckhardt (Naturhistorisches Museum Switzerland) for his contribution on insect identification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guedes, L.M., Aguilera, N., Ferreira, B.G. et al. Spatiotemporal variation in phenolic levels in galls of calophyids on Schinus polygama (Anacardiaceae). J Plant Res 132, 509–520 (2019). https://doi.org/10.1007/s10265-019-01118-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01118-6