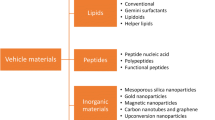
Abstract
Genetics and molecular genetic techniques have changed many perspectives and paradigms in medicine. Using genetic methods, many diseases have been cured or alleviated. Gene therapy, in its simplest definition, is application of genetic materials and related techniques to treat various human diseases. Evaluation of the trends in the field of medicine and therapeutics clarifies that gene therapy has attracted a lot of attention due to its powerful potential to treat a number of diseases. There are various genetic materials that can be used in gene therapy such as DNA, single- and double-stranded RNA, siRNA and shRNA. The main gene editing techniques used for in vitro and in vivo gene modification are ZNF, TALEN and CRISPR-Cas9. The latter has increased hopes for more precise and efficient gene targeting as it requires two separate recognition sites which makes it more specific and can also cause rapid and sufficient cleavage within the target sequence. There must be carriers for delivering genes to the target tissue. The most commonly used carriers for this purpose are viral vectors such as adenoviruses, adeno-associated viruses and lentiviruses. Non-viral vectors consist of bacterial vectors, liposomes, dendrimers and nanoparticles.
Graphical abstract





Similar content being viewed by others
References
Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675–85.
Oti M, Brunner HG. The modular nature of genetic diseases. Clin Genet. 2007;71(1):1–11.
Mulligan RC. The basic science of gene therapy. Science. 1993;260(5110):926–32.
Yu M, Poeschla E, Wong-Staal F. Progress towards gene therapy for HIV infection. Gene Ther. 1994;1(1):13–26.
Kumar R, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9(9):3362–8.
Tan J, et al. TERT promoter mutation determines apoptotic and therapeutic responses of BRAF-mutant cancers to BRAF and MEK inhibitors: Achilles Heel. Proc Natl Acad Sci. 2020;117(27):15846–51.
Da Vià MC, et al. CIC mutation as a molecular mechanism of acquired resistance to combined BRAF-MEK inhibition in extramedullary multiple myeloma with central nervous system involvement. Oncologist. 2020;25(2):112–8.
Verma IM, et al. Gene therapy: promises, problems and prospects. In: Genes and resistance to disease. Springer; 2000. p. 147–57.
Cavazzana-Calvo M, Thrasher A, Mavilio F. The future of gene therapy. Nature. 2004;427(6977):779–81.
Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–38.
Meyer F, Finer M. Gene therapy: progress and challenges. Cell Mol Biol. 2001;47(8):1277–94.
Maria BL, et al. Topical review: gene therapy for neurologic disease: benchtop discoveries to bedside applications. 2. The bedside. J Child Neurol. 1997;12(2):77–84.
Brown BD, Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood J Am Soc Hematol. 2002;100(4):1133–40.
Herzog RW, Cao O, Srivastava A. Two decades of clinical gene therapy–success is finally mounting. Discov Med. 2010;9(45):105.
Robinson A. Idecabtagene Vicleucel (Abecma®). Oncol Times. 2021;43(10):21.
Jaklevic MC. CAR-T therapy is approved for Non-Hodgkin Lymphoma. JAMA. 2021;325(11):1032–1032.
Reach T. FDA approves first oncolytic virus therapy: imlygic for melanoma. Oncol Times. 2015;37:36.
Philippidis A. Kymriah, First CAR-T Cancer immunotherapy approved by FDA. Mary Ann Liebert, Inc., NY; 2017
Prado DA, Acosta-Acero M, Maldonado RS. Gene therapy beyond luxturna: a new horizon of the treatment for inherited retinal disease. Curr Opin Ophthalmol. 2020;31(3):147–54.
Voelker R. CAR-T therapy is approved for mantle cell lymphoma. JAMA. 2020;324(9):832–832.
Papadouli I, et al. EMA review of Axicabtagene Ciloleucel (Yescarta) for the treatment of diffuse large B-cell lymphoma. Oncologist. 2020;25(10):894.
Nuijten M. Pricing Zolgensma–the world’s most expensive drug. J Mark Access Health Policy. 2022;10(1):2022353.
Ferreira GN, et al. Downstream processing of plasmid DNA for gene therapy and DNA vaccine applications. Trends Biotechnol. 2000;18(9):380–8.
Prather KJ, et al. Industrial scale production of plasmid DNA for vaccine and gene therapy: plasmid design, production, and purification. Enzym Microb Technol. 2003;33(7):865–83.
Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010;38(17):5797–806.
Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12(6):433–46.
Naveed A, et al. NEAT1 polyA-modulating antisense oligonucleotides reveal opposing functions for both long non-coding RNA isoforms in neuroblastoma. Cell Mol Life Sci. 2021;78(5):2213–30.
McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3(10):737–47.
Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci. 2003;100(17):9779–84.
Chakraborty C. Potentiality of small interfering RNAs (siRNA) as recent therapeutic targets for gene-silencing. Curr Drug Targets. 2007;8(3):469–82.
Taxman DJ, et al. Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. In: RNA therapeutics. Springer; 2010. p. 139–56.
Jiang F, Doudna JA. CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29.
Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308.
Fonfara I, et al. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42(4):2577–90.
Amitai G, Sorek R. CRISPR–Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol. 2016;14(2):67–76.
Weber T, et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol. 2015;33(5):543–8.
Bachu R, Bergareche I, Chasin LA. CRISPR-Cas targeted plasmid integration into mammalian cells via non-homologous end joining. Biotechnol Bioeng. 2015;112(10):2154–62.
Cho SW, et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24(1):132–41.
Maikova A, et al. Protospacer-adjacent motif specificity during clostridioides difficile type ib crispr-cas interference and adaptation. MBio. 2021;12(4):e02136-21.
Wan H, et al. Probing the behaviour of Cas1-Cas2 upon protospacer binding in CRISPR-Cas systems using molecular dynamics simulations. Sci Rep. 2019;9(1):1–16.
Huang TK, et al. Efficient gene targeting in Nicotiana tabacum using CRISPR/SaCas9 and temperature tolerant LbCas12a. Plant Biotechnol J. 2021;19:1314.
Haapaniemi E, et al. CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24(7):927–30.
Khalaf K, et al. CRISPR/Cas9 in cancer immunotherapy: animal models and human clinical trials. Genes. 2020;11(8):921.
Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188(4):773–82.
Urnov FD, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–46.
Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–47.
Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65(2):e26865.
Hoban MD, et al. Delivery of genome editing reagents to hematopoietic stem/progenitor cells. Curr Protoc Stem Cell Biol. 2016;36(1):4.1-4.10.
Bedell VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–8.
Xia E, et al. TALEN-mediated gene targeting for cystic fibrosis-gene therapy. Genes. 2019;10(1):39.
Dunbar CE, et al. Gene therapy comes of age. Science. 2018;359(6372):eaan4672.
Gardlík R, et al. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11(4):110–21.
Maguire CA, et al. Gene therapy for the nervous system: challenges and new strategies. Neurotherapeutics. 2014;11(4):817–39.
Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6(2):42.
Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80(1):35–47.
Yi Y, Hahm SH, Lee KH. Retroviral gene therapy: safety issues and possible solutions. Curr Gene Ther. 2005;5(1):25–35.
Yi Y, Jong Noh M, Hee Lee K. Current advances in retroviral gene therapy. Curr Gene Ther. 2011;11(3):218–28.
Takeuchi Y. Gene therapy using retrovirus vectors: vector development and biosafety at clinical trials. Uirusu. 2015;65(1):27–36.
Palu G, et al. Progress with retroviral gene vectors. Rev Med Virol. 2000;10(3):185–202.
Cannon PM, et al. Murine leukemia virus-based Tat-inducible long terminal repeat replacement vectors: a new system for anti-human immunodeficiency virus gene therapy. J Virol. 1996;70(11):8234–40.
Suerth JD, et al. Self-inactivating alpharetroviral vectors with a split-packaging design. J Virol. 2010;84(13):6626–35.
Maier P, Von Kalle C, Laufs S. Retroviral vectors for gene therapy. Future Microbiol. 2010;5(10):1507–23.
Onodera M, et al. Gene therapy for severe combined immunodeficiency caused by adenosine deaminase deficiency: improved retroviral vectors for clinical trials. Acta Haematol. 1999;101(2):89–96.
Enquist IB, et al. Effective cell and gene therapy in a murine model of Gaucher disease. Proc Natl Acad Sci. 2006;103(37):13819–24.
Herrera-Carrillo E, Berkhout B. Bone marrow gene therapy for HIV/AIDS. Viruses. 2015;7(7):3910–36.
Narayan O, Clements JE. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989;70(7):1617–39.
Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9(5):457–63.
Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp. 2010;58(2):107–19.
Smith JG, et al. Adenovirus. Cell Entry Non-Enveloped Viruses. 2010; 195–224.
Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors. Appl Biochem Biotechnol. 2006;133(1):9–29.
Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132(1–2):1–14.
McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15(11):1022–33.
Berns KI, Bohenzky RA. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306.
Gao G, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci. 2003;100(10):6081–6.
Berns K, Giraud C. Biology of adeno-associated virus. In: Adeno-Associated Virus (AAV) vectors in gene therapy. Springer; 1996. p. 1–23.
Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–93.
Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. In: Clinical infectious diseases. JSTOR; 1998. p. 541–53.
Andreansky SS, et al. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci. 1996;93(21):11313–8.
Aurelian L. Herpes simplex viruses. In: Clinical virology manual. 4th ed. American Society of Microbiology; 2009. p. 424–53.
Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci. 1996;93(21):11307–12.
Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2(12):938.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62.
Lawler SE, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–9.
Woller N, et al. Oncolytic viruses as anticancer vaccines. Front Oncol. 2014;4:188.
Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9.
Trager MH, Geskin LJ, Saenger YM. Oncolytic viruses for the treatment of metastatic melanoma. Curr Treat Options Oncol. 2020;21(4):1–16.
Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254(2):178–216.
Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89(1):21–39.
Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4(3):218–27.
Amer MH. Gene therapy for cancer: present status and future perspective. Mol Cell Ther. 2014;2(1):1–19.
El-Aneed A. Current strategies in cancer gene therapy. Eur J Pharmacol. 2004;498(1–3):1–8.
Hambleton S. Chickenpox. Curr Opin Infect Dis. 2005;18(3):235–40.
Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–9.
Bellini WJ, Rota JS, Rota PA. Virology of measles virus. J Infect Dis. 1994;170(Supp 1):S15–23.
Blechacz B, Russell SJ. Measles virus as an oncolytic vector platform. Curr Gene Ther. 2008;8(3):162–75.
Russell SJ, Peng KW. Measles virus for cancer therapy. In: Measles. Springer; 2009. p. 213–41.
Ganar K, et al. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81.
Zhao H, Peeters BP. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J Gen Virol. 2003;84(4):781–8.
Burman B, Pesci G, Zamarin D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers. 2020;12(12):3552.
Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 2001;6(1):66–77.
Caracciolo G, Amenitsch H. Cationic liposome/DNA complexes: from structure to interactions with cellular membranes. Eur Biophys J. 2012;41(10):815–29.
Stewart MJ, et al. Gene transfer in vivo with DNA–liposome complexes: safety and acute toxicity in mice. Hum Gene Ther. 1992;3(3):267–75.
Masotti A, et al. Comparison of different commercially available cationic liposome–DNA lipoplexes: parameters influencing toxicity and transfection efficiency. Colloids Surf B. 2009;68(2):136–44.
Tseng W-C, Huang L. Liposome-based gene therapy. Pharm Sci Technol Today. 1998;1(5):206–13.
Bendas G. Immunoliposomes. BioDrugs. 2001;15(4):215–24.
Paszko E, Senge M. Immunoliposomes. Curr Med Chem. 2012;19(31):5239–77.
Zhang X-X, McIntosh TJ, Grinstaff MW. Functional lipids and lipoplexes for improved gene delivery. Biochimie. 2012;94(1):42–58.
de Ilarduya CT, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–70.
Jewell CM, Lynn DM. Surface-mediated delivery of DNA: cationic polymers take charge. Curr Opin Colloid Interface Sci. 2008;13(6):395–402.
Hwang S, Davis M. Cationic polymers for gene delivery: designs for overcoming barriers to systemic administration. Curr Opin Mol Ther. 2001;3(2):183–91.
Vermeulen LM, et al. The proton sponge hypothesis: fable or fact? Eur J Pharm Biopharm. 2018;129:184–90.
Boas U, Heegaard PM. Dendrimers in drug research. Chem Soc Rev. 2004;33(1):43–63.
Navarro G, DeILarduya CT. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed Nanotechnol Biol Med. 2009;5(3):287–97.
Mohanraj V, Chen Y. Nanoparticles—a review. Trop J Pharm Res. 2006;5(1):561–73.
Tian H, Chen J, Chen X. Nanoparticles for gene delivery. Small. 2013;9(12):2034–44.
Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149(1):65–71.
Baban CK, et al. Bacteria as vectors for gene therapy of cancer. Bioengineered bugs. 2010;1(6):385–94.
Lin D, et al. Bacterial-based cancer therapy: an emerging toolbox for targeted drug/gene delivery. Biomaterials. 2021;277:121124.
Xiang S, Fruehauf J, Li CJ. Short hairpin RNA–expressing bacteria elicit RNA interference in mammals. Nat Biotechnol. 2006;24(6):697–702.
Bernardes N, Chakrabarty AM, Fialho AM. Engineering of bacterial strains and their products for cancer therapy. Appl Microbiol Biotechnol. 2013;97(12):5189–99.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2(2):141.
Nikolaou M, et al. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metas. 2018;35(4):309–18.
Aleksakhina SN, Kashyap A, Imyanitov EN. Mechanisms of acquired tumor drug resistance. Biochim Biophys Acta Rev Cancer. 2019;1872:188310.
Dominiak A, et al. Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers. 2020;12(5):1232.
Asiry S, et al. The cancer cell dissemination machinery as an immunosuppressive niche: a new obstacle towards the era of cancer immunotherapy. Front Immunol. 2021;12:654877.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299–309.
Wu D, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Seminars in cancer biology. Elsevier; 2017.
Wang Y, et al. Nucleolin-targeted extracellular vesicles as a versatile platform for biologics delivery to breast cancer. Theranostics. 2017;7(5):1360.
Yao C, et al. Reducible self-assembling cationic polypeptide-based micelles mediate co-delivery of doxorubicin and microRNA-34a for androgen-independent prostate cancer therapy. J Control Release. 2016;232:203–14.
Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):1–12.
Krzyszczyk P, et al. The growing role of precision and personalized medicine for cancer treatment. Technology. 2018;6:79–100.
Barthélémy F, Wein N. Personalized gene and cell therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28(10):803–24.
Morash M, et al. The role of next-generation sequencing in precision medicine: a review of outcomes in oncology. J Personal Med. 2018;8(3):30.
Siena S, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29(5):1108–19.
De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4(8):662–74.
Ichikawa H, et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017;9(1):1–12.
Chatzopoulou F, et al. Dissecting miRNA–gene networks to map clinical utility roads of pharmacogenomics-guided therapeutic decisions in cardiovascular precision medicine. Cells. 2022;11(4):607.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
M.D. wrote the main manuscript text, prepared figures and tables, edited the manuscript and reviewed it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The author declares that there is no potential conflict of interest related to this research and publication.
Human or animal rights
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danaeifar, M. Recent advances in gene therapy: genetic bullets to the root of the problem. Clin Exp Med 23, 1107–1121 (2023). https://doi.org/10.1007/s10238-022-00925-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00925-x