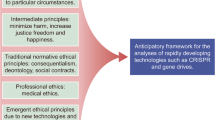
Abstract
The field of genetics has seen major advances in recent decades, particularly in research, prevention and diagnosis. One of the most recent developments, the genomic editing technique Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9, has opened the possibility for genetic therapies through genome modification. The technique marks an improvement on previous procedures but poses some serious ethical conflicts. Bioethics is the discipline geared at finding answers to ethical challenges posed by progress in medicine and biology and examining their repercussions for society. It can also offer a conceptualization of these ethical dilemmas. The aim of this paper is to offer a map of the ethical dilemmas associated with this technique by way of a critical analysis of current literature. The main issues can be grouped in four areas: efficacy and security; the types of cells which can be targeted by the technique (somatic, embryonic and gametes); the goal of the therapy; and accessibility and justice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The field of genetics has undergone remarkable development in recent years, and promising advances are constantly being made. One example of this is the recent development of the Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 genome editing technique, which has made the modification of the human genome a distinct possibility. This is reminiscent of the news of the birth of two babies genetically modified in 2018 by the Chinese scientist He Jiankui.
These new possibilities raise ethical questions about this technique that have important implications for individuals, society and indeed the whole human species. Literature on the technical characteristics of CRISPR and the ethical challenges that it poses is already abundant, but the rapid pace of progress makes it difficult to establish a clear and precise view of the challenges that it poses.
Bioethics is the discipline geared at finding answers to ethical challenges posed by progress in medicine and biology. Its aim is to discuss the problems posed by medical and biological advances and the impact that they have on society and its value systems Abel [1].
As Bauman [2] observed: ‘Ethics…must deal with what-has-not-happened-yet, with a future that is endemically the realm of uncertainty and the playfield of conflicting scenarios. Visualization can never pretend to offer the kind of certainty which experts with their scientific knowledge and with greater or lesser credibility claim to offer. The duty to visualize the future impact of action (undertaken or not undertaken) means acting under the pressure of acute uncertainty. The moral stance consists precisely in seeing to it that this uncertainty is neither dismissed not suppressed, but consciously embraced’. This is not about preventing progress, but rather about being able to visualize the impact of our actions and thereby to minimize any possible negative effects.
This article aims to present a map of all the ethical issues raised by the CRISPR technique and to succinctly reflect upon them. The literature on the ethical challenges posed by CRISPR is already plentiful, but most of the articles published to date have tended to focus on only a limited number of these issues (and especially on those related to technical questions). We aim to present a global overview of all of the issues that arise (including technical, anthropological and social considerations). It should be noted that some of these arise in almost all genome editing techniques and are not exclusive to CRISPR; even so, CRISPR may cause them to surface in an easier, quicker or more intense way [3].
This work is the product of a project on the ethical implications of using the CRISPR technique that was developed by a multi-disciplinary group of researchers working at our institute. It is based on previous work, published in Medicina Clínica [4], whose objective was limited to making a brief enumeration of these issues (but without examining them in depth). Their mapping was, however, able to identify and define a series of potential conflicts and made it possible to analyse and provide a response to each of them, either in the form of regulation or a moratorium.
In presenting this global overview, the article addresses the following topics: the characteristics of the technique and its application; related ethical issues; the uses and purposes of the technique; and, finally, the question of social justice.
Characteristics of the Technique
The CRISPR technique is a genetic editing procedure that was first used in 2012 by a group of researchers from the University of Berkeley.Footnote 1 There are also other, similar, editing techniques (based on recombinant DNA), such as TALEN (transcription activator-like effector nucleases) and ZFN (zinc-finger nucleases), but CRISPR provides a better combination of three key factors: precision, accessibility and price. It is also easier to use than the alternatives, as the other techniques require more time and more specialised personnel.
It is a procedure that starts with the DNA of bacteria that provide an immune mechanism against viruses. These sequences are able to recognize viruses that enter bacteria and which ‘activate’ an enzyme that is able to break them down. In doing this, the enzyme makes use of the resulting fragments to immunise the bacteria against the virus. This process is made possible by CRISPR (the NRA molecule which transmits the biological information contained in the DNA for protein synthesis) using Cas9, which is a specific enzyme belonging to bacteria that can repair fragments pf DNA with great precision [5, 6].
Application of the Technique
Genetic editing (and, in particular, the CRISPR technique) can be used in people, animals and plants [7, 8].
In animals, genetic editing [9] can be used in food production (to increase muscle mass, improve nutritional content and breed more manageable animals) and to avoid, or prevent, diseases that could affect humans (for example, genetically modifying vectors to eradicate disease, as in the case of the Aedes aegypti mosquito, which transmits dengue fever, or in certain subspecies of the Anopheles mosquito genus, which carry the malaria parasite). Another hypothetical, although as-of-yet unfeasible, use of the technique would be to obtain organs to transplant into humans. In plants, genetic editing has also been used to improve food destined for human or animal consumption.
Some of the ethical problems associated with using this technique in animals and plants involve: potentially causing significant transformations of insect or plant species that could alter important ecological balances; producing ‘off-target’Footnote 2 effects that may not be possible to control; having effects on animals and people that consume genetically modified animals; and the risk of unnecessarily and irresponsibly reducing the level of biodiversity [9, 10].
Genetic Editing in Humans
The application of genetic editing techniques to humans is undoubtedly one of the issues that causes most debate and ethical interest in genetic engineering [3]. It has, however, been presented as one of the best tools for potentially avoiding, or preventing, diseases, as well as for genetically modifying an organism.
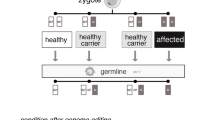
For example, certain types of cancer are currently treated using gene editing in somatic cells and there are on-going trials to treat Cooley's anaemia (β-thalassemia), sickle cell anaemia, mucopolysaccharidosis (types I and II) and haemophilia B, amongst other pathologies. For example, in 2017, S. Mitalipov and his team used the CRISPR technique to ‘repair’ a mutation associated with hypertrophic cardiomyopathy in an ovule, just prior to fertilisation [11]. The mutation was not ‘inherited’ by the resulting zygote and no mosaicismsFootnote 3 or ‘off-target’ effects were observed [12,13,14,15].
There are three different types of cells which can be modified by genetic editing, with different repercussions for the subjects:
-
1.
Somatic cells: Their genetic modification only affects the individual, not their offspring.
-
2.
Embryonic (pluripotential) cells: Their genetic modification only tends to affect the individual (although, in some cases, it can also affect their offspring).
-
3.
Gametes: Their genetic modification affects the individual and is also transmitted to their offspring [10].
When possible, germ-line modification (which alters the genetic inheritance) can be achieved in two ways: by modifying germ cells (gametes: sperm cells or oocytes /ovules) and by modifying the zygote, or embryo, at an early stage in its development: before the formation of its reproductive organs [12].
Map of Ethical Conflicts in the Genetic Editing Technique
The European Society of Human Reproduction and Embryology (ESHRE) and the European Society of Human Genetics (ESHG) issued a document early in 2018, which was subsequently followed by another, setting out their rationale and outlining a series of practical recommendations. In this way, they officially stated their position regarding the ethical questions posed by the new technique [12, 13]. Both documents particularly focused on issues related to the genetic editing of germ cells.
Amongst the various problems, or ethical issues, raised by genetic editing using the CRISPR technique, it is possible to distinguish four different groups. The first is associated with the technique itself and its effectiveness and safety; the second is related to the type of cells to which the technique is applied; the third refers to the purpose for which it is applied; and the fourth is related to its accessibility. Although these different groups of issues are closely related, they are not really the same in nature.
Ethical Issues Related to the Technique
The most important ethical problems presented by this technique are surely those related to its safety and efficacy [15]. With respect to precision, it should be underlined that this approach is not as accurate as might be expected [10, 15, 16]. Guttinger [6] states that total accuracy is not possible due to the complexity of DNA sequencing and its relationship with the RNA molecule.
Another difficult issue is related to the efficiency of the technique and the difficulty involved in controlling and determining its off-target effects. If its accuracy were total, and if it were possible to always intervene on the desired gene, the next problem would be how to determine whether the effects of the intervention were, in fact, only the ones sought [6, 10, 15, 16]. CRISPR is the gene editing technique that has the most off-target effects (compared to TALEN and ZFN) [15], although efforts are currently being made to reduce this unpredictability [10].
Such problems of safety and efficacy could also be present in He Jiankui’s experiments (He [17], according to two articles published in December 2019, in the MIT Technology Review [18, 19]). Regalado [19] has explained the trajectory of the publication of He’s work ‘Birth of Twins After Genome Editing for HIV Resistance’ in several scientific journals and highlighted some serious methodological and ethical irregularities. Having reviewed He Jiankui’s work, Musurunu (2019) stated that evidence of mosaicism was present in both twin embryos, so they could still have been vulnerable to HIV, and that the possible presence of off-target mutations could not be completely ruled out. The negative impact of He Jiankui’s experiments may not only have caused damage to those directly affected, but it could also have slowed down research into genetic editing and in similar fields [20, 21].
As a result, more knowledge is required before this technique should be applied to humans. For genetic editing to be successful, it is necessary to know how to determine the impact of small changes in DNA (or its ‘packaging’) on the chemical components and physical properties of cells. It is, therefore, important to improve our existing knowledge of genetic and epigenetic effects in order to subsequently determine, and predict, the phenotypic effects of genetic editing [15].
Bearing in mind these shortcomings, O’Keefe, Perrault and other researchers have asked whether it would not be a good idea to change the language used (and the metaphors reflected within it) when describing, or discussing, the CRISPR technique, and especially when referring to it in the press and other non-specialised types of literature. In fact, in the video produced by He Jiankui [17], the most repeated words are ‘safely’ and ‘healthily’. The verbs most frequently used to describe the process (‘edit’, ‘cut’, ‘erase’ and ‘repair’) suggest a degree of accuracy and security that, in practice, do not exist,or, at least, not to such a high degree. This is why authors often advocate using terms like ‘modify’, ‘change’ and ‘alter’, which are more realistic and have fewer potentially misleading connotations [22].
Ethical Issues Related to Its Use
As previously mentioned, there are three types of cells that can be the object of genetic editing: somatic cells, embryonic (pluripotential) cells and germ cells (gametes). Somatic cells, and most embryonic cells, present the fewest ethical problems since the intervention upon them only affects the individual, but not their offspring. In these cases, the main ethical criteria to consider are non-maleficence, the ratio between risk and benefit, and consent.
In the case of interventions on germline cells, any modifications should (when technically possible) be carried out in one of two ways: by modifying the germ cells (gametes: sperm cells or oocytes) or by modifying the zygote, or embryo, at an early stage [15].
Interventions involving germline cells could potentially affect offspring. Any modification, where feasible, could, however, be carried out in one of two ways: by modifying the germ cells (gametes: sperm or oocytes) or by modifying the zygote or the embryo at an early stage [15].
Liang, Xu, Zhang, Ding and several other Chinese scientists first applied the CRISPR technique to embryos in 2015. A year later, another group of Chinese researchers (Kang, He, Huang and others) repeated the process [22]. Both experiments involved applying the technique to in vitro fertilized zygotes that were defective and unviable for reproductive implantation (because they were triploid). Neither of the projects was successful, and they were characterised by imprecision, mosaicism and numerous ‘off-target’ mutations [8, 12].
Both projects highlighted the technical and ethical problems of genetic editing in the germline. From a technical point of view, there are basically three types of embryos that can be investigated: (1) those that are not viable or which are unsuitable for fertilization treatments; (2) viable/adequate but leftover embryos; and (3) embryos created specifically for research [10].
Genetic research is currently performed on embryos of the second and third types: embryos with a high probability of mosaicism. The specific creation of embryos for research purposes is not legally permitted in most countries [12].
However, even if access to optimal zygotes and embryos were possible, there are still certain technical obstacles that would have to be overcome. The possibility of analysing the genetically modified embryo is limited, since this analysis would have to be carried out during the subsequent in vitro culture period; this would impose limitations in terms of both technique and time. In addition, reducing the usable embryos to those obtained from in vitro fertilization processes, as opposed to being able to specifically create embryos for research, would reduce the quantity and quality of the embryos availableFootnote 4 [23].
These types of technical limitations involve primary ethical issues. As previously stated, as of today, a completely safe and precise genetic alteration of the germline is effectively impossible. To achieve this, better knowledge and better technology are required, and this would call for more research to be carried out. Secondary ethical issues relate to the very use of embryos in scientific research.
There are some scientists who believe that research with human embryos is not ethically acceptable because it involves dealing with organisms, or entities, with human status or which have dignity [5, 10]. Others, including members of the ESHRE and ESHG, argue that the embryo has a lower moral ‘status’ than the foetus which, in turn, has a lower moral ‘status’ than a child or adult. The ESHRE and ESHG have no objections to using discarded, or leftover, embryos for research [12]. Along the same lines, Savulescu et al. [24] argue that the main consideration concerning genetic experimentation on embryos (and, more specifically, the use of the CRISPR technique) is that they would not be useful in any other way.
Similarly, the ESHRE and ESHG see no problems with and have no moral objections to the creation of embryos explicitly and directly for research purposes, since their moral status is the same as that of embryos that have been left over from, or discarded during, in vitro fertilization processes [12].
There are some researchers, however, who point out that any use of embryos for research, regardless of their origin, runs the risk of falling upon a slippery slope: Accepting certain practices will easily open the door to others that are regarded as unacceptable. It should be noted that the ethical problem is not the ‘slippery slope’ itself, but rather these potentially unacceptable practices. Along these lines, several authors have suggested that the following limitations should be accepted by the entire scientific community (as has already occurred in the USA, China, the UK and Sweden): no investigating with embryos that are more than 14 days old and no implantations for reproduction (in any species) of any embryos that have already been used in research [10].
The genetic modification of germ cells consists of the application of genetic editing techniques in the process of gametogenesis: their application to original, or primordial, cells that will engender sperm and the mature ovum [25]. The female germ cell is more accessible, and susceptible, to genetic editing, but it is not yet possible to act upon the previous, or precursor, cell of the oocyte (the immature precursor of the ovum), and there is controversy in this regard [12].
Another option would be to produce in vitro gametes from pluripotential stem cells, but this technique has yet to be fully developed in animals [12]. As a result, the genetic editing of this type of cell is currently impossible in humans, even using the CRISPR technique. This should be mentioned as a research channel, however, because it is considered a possible future option.
Having already mentioned the use of embryos in experimentation, it is necessary to underline some other ethical issues that may help to configure the map of ethical conflicts associated with the genetic modification of the germline.
Although this article analyses the issue from an ethical point of view, to fully address this question, it is also important to make reference to the legal framework. From the legal perspective, at least in Europe, this matter is not subject to discussion and there is no leeway for different possibilities of implementation. This is because, on the one hand, the European Convention on Human Rights and Biomedicine (Convention of Oviedo; Article 13 (1997)) strictly prohibits any modification of the human genome that could affect their offspring and, on the other, the European regulation governing clinical trials (EU Regulation No. 536/2014) does not allow any type of genetic experimentation that could alter the subject’s germ line genetic identity [12]. However, not all European countries have ratified the Oviedo Convention [26] and, moreover, within the field of ethics, there is no general agreement amongst the scientific community as to the use that should be made of the CRISPR technique in genetic experimentation [27].Footnote 5
Both the ESHRE and ESHG consider that no theoretical ethical objections should prevent accepting germline gene modification. They do, however, recognize the previously mentioned technical objections related to safety and precision, avoiding off-target effects and anticipating the consequences of genetic modifications on subsequent generations. Overcoming these limitations would require more research, and in the opinion of these institutions, it would be ethically unacceptable to attempt any modifications in germline genetics until this has been completed [12, 27].
Other authors do not consider the unpredictable impact that (at least some of) these consequences could have on future generations to be sufficient, in themselves, to warrant a solid moral objection. This is, for example, a thesis supported by Sugarman and Savulescu, who argue that it is not, in fact, possible to do research into science and technology while knowing, or being able to anticipate, all of the effects that a given discovery, or technique, may have on future generations [24, 28].
At the same time, several authors have stated that it would raise a fundamental moral problem if any alteration to the germline were to imply converting future generations into effective research participants. This would make it necessary to monitor, or even control, its development. The ‘participants’ in that control group would always need to give their informed consent which, for obvious reasons, could not possibly be obtained at the time of starting the trial. Any genetic modification to the germline that could cause effects that would be transmissible to offspring would therefore require monitoring during subsequent generations [29]. However, the reply to that objection is that such a limitation would, in fact, effectively constitute a limitation to any type of research that could directly, or significantly, affect members of future generations. It should be added that it is never possible to request the consent of all the individuals who are directly involved in research initiated by a previous generation [13, 24, 28]. However, in this particular case, the involvement of subjects without their consent would be direct.
Mintz [30] argued that although the embryo does not have the capacity for autonomous decision-making at the time of the germline engineering, the decision taken by the parents could affect its future autonomy. Citing Feinberg [31], he argued that to protect the autonomy of future children, parents should be helped to make ethical decisions that would give their children an open future.
The ethical debate could be summarized in relation to the four questions, or criteria, put forward by Lehmann [14] for morally assessing any genetic modification (whether in the germ line, or not). The author called these criteria the ‘4-S’: (1) Safety, (2) the Significance of the harm to be avoided or averted, (3) Successive generations and (4) Social consequences.
Hildt [23] argued that any attempt to genetically modify the germline should meet three conditions that do not tend to be met at present:
-
1.
Previous solid and safe experience in gene therapy with somatic cells
-
2.
Tests on animals that would guarantee safety and reproducibility and suggest that any subsequent human interventions would be successful
-
3.
Public approval (social, political…) of the technique
The criteria mentioned in the last two points have several social implications that must be considered when evaluating the moral permissibility of genetic research. In particular, these are relevant to actions that involve changes in the germline. These social consequences are not usually included amongst the criteria considered in the literature, but we think that they have a specific weight in the moral debate.
Ethical Issues Related to Its Purpose
To expand the map of ethical issues related to genetic editing and the use of the CRISPR technique, one fundamental question within the debate is its purpose. There are, or can be, two main reasons for genetic editing:
-
The first is therapy: the treatment or prevention of a given disease
-
The second is improvement: modifying a characteristic (or several characteristics) of an individual (or species) that is (or are) not a disease
The debate and questions about these two points could be expressed in terms of the following questions: (1) Is a genetic modification ethically acceptable when performed for therapeutic purposes: to cure or prevent disease? And (2) Is it ethically acceptable to produce a change in a patient when what is modified (or improved) does not constitute or involve a disease.
Lehmann has posed the following questions related to this debate: (1) Should humans use genetic editing not only to prevent disease, but also to improve a given individual, or indeed, the whole species (for example by helping them to adapt to climate change)? (2) If this is the case, who should have the power to control, or decide, this and also how to distribute the available resources? (3) Should parents be able to decide whether to genetically modify their children (at the embryonic stage) and to take decisions that would affect them (and future generations) for as long as they live? [14].
Agar [32] suggests that we should distinguish between ‘morally wrong’ practices, which should be condemned, and ‘morally problematic’ practices, which call for ‘solutions’. According to Agar, genetic editing in order to make improvements would fall into the latter ‘problematic’ category, with the issue being complex, but not necessarily ‘wrong’.
Broadly speaking (and without entering into intermediate nuances), there are two opposing positions here: Some argue that genetic editing is only ethically acceptable to cure, or prevent, disease, while others argue that, in some cases, gene editing for human enhancement is not only acceptable, but even a moral duty.
The argument put forward by advocates of improvement is that medicine is already used to 'improve' individuals and species (for example, through vaccines and surgery). They also argue that there could even be a moral obligation to improve the human body and health, if the means are available to do so [14, 24]. In contrast, others hold that the use of genetics or genomic modification to improve non-pathological features of individual humans (and the species as a whole) could easily lead to eugenic practices and to the value of the individual being given less importance than certain bodily and cognitive characteristics [5].
This reflection on human improvement poses important questions whose scope goes beyond genetics and the CRISPR technique but which could have a powerful influence upon them, both now and in the future. Check raises several of these questions: (1) Which diseases should be eradicated or prevented? (2) Should all disabilities be eradicated? (3) In fact, what is a disease? (4) Is it ethically and socially good to eradicate everything that is judged to be ‘defective’ (or any feature considered to be so)? [33].
Ethical Issues Related to Justice
In health care and scientific research, one of the main principles, or fundamental ethical criteria, is that of justice. This principle establishes, amongst other duties, the obligation to always consider the social consequences of technological and scientific ‘advances’. Paradoxically, these social consequences are a factor that the scientific literature does not usually consider. In the case of genetic editing techniques, in general, and the CRISPR technique, in particular, these relate to questions concerning accessibility, equality and representativeness.
The financial cost of therapy is a fundamental factor when considering the question of justice. Marketing an expensive therapy could effectively exacerbate existing inequalities in health provision, in both poor and rich countries (an example is the USA, with its marked inequalities in the reception of health) [34,35,36]. On the other hand, in the public health systems of Western countries, the problem would be the impact of its cost for the health system, which should not threaten the sustainability of the whole system. Such inequalities could, as Bellver [5] has pointed out, cause a genetic alteration of the germline that would potentially generate excessive inequalities between different individuals, communities and societies, giving rise to what has been referred to as aristocracy genetics.
Likewise, the inclusion and representativeness of all sectors of the population is also important, as in the case of minorities (such as different ethnic groups) [35]. When engaging in debate and considering the scope of such hypothetical genetic alterations, the diversity of the genetic sequencing found in certain individuals, or groups, within the human population should not be placed in jeopardy.
Conclusion
The CRISPR genetic editing technique is a technological procedure that, while improving on previous approaches, is not, in itself, exempt from certain ethical problems (some of which are technical). The scientific literature has already focused on some of these, but there is still a relative lack of publications highlighting the full range of this problem. It can, however, be concluded that the map of ethical problems associated with this technique should consider the following points:
-
Efficiency and safety: Efficiency and safety are two considerations that have not, to date, received as much attention as might be expected.
-
Cell type: The types of cells that are, or may be used, in this technique, and the applications that it may be given, are the subject of a moral debate. The ethical questions related to somatic cell interventions are not the same as those associated with germ cell interventions. The use of embryos should also be debated in a similar way (whether it is ethical for them to be subject to research, or the application of the technique, and which types of embryos can be used, etc.).
-
Purpose: From the perspective of morality, the implications are not the same when the purpose of using the technique is associated with therapy to treat certain diseases, as when the objective is to improve certain aspects, or traits, that are not considered ‘pathological’ (whether these apply to individuals, or to the human species as a whole).
-
Justice: It is also important to consider the social impact that extending the application of the technique could have regarding accessibility, costs, possible inequalities and the position of ethnic or social minorities.
Rosenbaum outlined the need to establish a social consensus [37] and to perhaps declare a moratorium. The conceptualization of a map of ethical conflicts, of the type presented in this article, clearly defines the different problem areas, helps to define a reference framework for debate and establishes positions and a base for subsequent legal developments.
Notes
It should be pointed out that the technique had already been successfully used, but that had been for editing DNA [2].
Mosaicism is a genetic alteration, whereby cells with different genotypes coexist in the same organism. In embryos, they arise due to a fault in the nuclease ruptures. They are the result of either the imprecise reparation of DNA before the embryo has begun its cell-division stage [12] or the onset of cell-division preceding any form of genetic editing [27].
For example, Savulescu points out that hundreds, or even thousands, of embryos are needed to run the experiments required for research into polygenic diseases [6].
Hence, in July 2017, Chneiweiss, Hirsch, Montoliu and other researchers (representing over 20 institutions) proposed creating a European Steering Committee to analyse and evaluate the advantages and dangers of the new technique [23].
References
Abel I, Fabre F (2001) Bioética: Orígenes, presente y futuro. Fundación Mapfre IBB, Barcelona
Bauman Z (1993) Postmodern ethics. Wiley-Blackwell, Oxford
Baumann M (2016) CRISPR/Cas9 genome editing - New and old ethical issues arising from a revolutionary technology. NanoEthics 10:139–159
Lorenzo D, Esquerda M (2019) Mapa de conflictos éticos de la técnica de edición genética CRISPR-Cas9. Med Clin 153(9):357–359
Bellver V (2016) La revolución de la edición genética mediante CRISPR-Cas9 y los desafíos éticos y regulatorios que comporta. Cuad Bioet 27(2):223–239
Guttinger S (2018) Trust in science: CRISPR–Cas9 and the ban on human germline editing. Sci Eng Ethics 24(4):1077–1096. https://doi.org/10.1007/s11948-017-9931-1
Camporesi S, Cavaliere G (2016) Emerging ethical perspectives in the clustered regularly interspaced short palindromic repeats genome-editing debate. Pers Med 13(6):575–586. https://doi.org/10.2217/pme-2016-0047
Dzau VJ, Cicerone RJ (2015) Responsible use of human gene-editing technologies. Hum Gene Ther 26(7):411–412. https://doi.org/10.1089/hum.2015.29004.vjd
Caplan AL, Parent B, Shen M, Plunkett C (2015) No time to waste—The ethical challenges created by CRISPR. EMBO Rep 16(11):1421–1426. https://doi.org/10.15252/embr.201541337
Plaza A, Lanner F (2017) Towards a CRISPR view of early human development: Applications, limitations and ethical concerns of genome editing in human embryos. Development 144(1):3–7. https://doi.org/10.1242/dev.139683
Ma H, Marti-Gutierrez N, Park SW et al (2017) Correction of a pathogenic gene mutation in human embryos. Nature 548:413–419. https://doi.org/10.1038/nature23305
De Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, Van El CG, Rial-Sebbag E (2018) Responsible innovation in human germline gene editing. Background document to the recommendations of ESHG and ESHRE. Hum Reprod Open 1 hox024.
De Wert G, Pennings G, Clarke A, Eichenlaub-Ritter U, Van El CG, Forzano F, Rial-Sebbag E (2018) Human germline gene editing. Recommendations of ESHG and ESHRE. Eur J Hum Genet 26(4):445–449. https://doi.org/10.1038/s41431-017-0076-0.
Lehmann LS (2017) Is editing the genome for climate change adaptation ethically justifiable? AMA J Ethics 19(12):1186–1192. https://doi.org/10.1001/journalofethics.2017.19.12.stas1-1712
Tauxe W (2015) 4 big questions. Nature 528(7580):S17–S17. https://doi.org/10.1038/528S17a
De Lecuona I, Casado M, Marfany G, Baroni ML, Escarrabill M (2017) Focus: Genome editing: Gene editing in humans: Towards a global and inclusive debate for responsible research. Yale J Biol Med 90(4):673
He J (2018) About Lulu and Nana: Twin girls born healthy after gene surgery as single-cell embryos [Video File]. Retrieved from https://www.youtube.com/watch?v=th0vnOmFltc
Kusunuru K (2019) We need to know what happened to CRISPR twins Lulu and Nana, MIT Technol Rev. Retrieved from www.technologyreview.com/s/614762/crispr-baby-twins-lulu-and-nana-what-happened/
Regalado A (2019) China’s CRISPR babies: Read exclusive excerpts from the unseen original research, MIT Technol Rev. Retrieved from www.technologyreview.com/s/614764/chinas-crispr-babies-read-exclusive-excerpts-he-jiankui-paper/
Li H, Yin S (2020) Affected genome editing crops: The consequences of genome-edited babies in China. Sci Eng Ethics 1–4
Normile D (2019) China tightens rules on gene editing. Science 363(6431):1023
O’Keefe M, Perrault S, Halpern J, Ikemoto L, Yarborough M, UC North Bioethics Collaboratory for Life & Health Sciences (2015) “Editing” genes: A case study about how language matters in bioethics. Am J Bioethics 15(12):3–10. https://doi.org/10.1080/15265161.2015.1103804
Hildt E (2016) Human germline interventions–Think first. Front Genet 7:81. https://doi.org/10.3389/fgene.2016.00081
Savulescu J, Pugh J, Douglas T, Gyngell C (2015) The moral imperative to continue gene editing research on human embryos. Protein Cell 6(7):476–479. https://doi.org/10.1007/s13238-015-0184-y
Church G (2017) Compelling reasons for repairing human germlines. N Engl J Med 377(20):1909–1911. https://doi.org/10.1056/NEJMp1710370
De Wert G, Heindryckx B, Pennings G Clarke A, Eichenlaub-Ritter U, van El CE, Forzano F, Goddijn M, Howard HC, Radojkovic D, Rial-Sebbag E, Dondorp W, Tarlatzis BC, Cornel MC (on behalf of the European Society of Human Genetics and the European Society of Human Reproduction and Embryology) (2018) Responsible innovation in human germline gene editing: Background document to the recommendations of ESHG and ESHRE. https://doi.org/10.1038/s41431-017-0077-z
Chneiweiss H, Hirsch F, Montoliu L, Müller AM, Fenet S, Abecassis M, Kritikos M (2017) Fostering responsible research with genome editing technologies: A European perspective. Transgenic Res 26(5):709–713. https://doi.org/10.1007/s11248-017-0028-z
Sugarman J (2015) Ethics and germline gene editing. EMBO Rep 16(8):879–880. https://doi.org/10.15252/embr.201540879
Cwik B (2017) Designing ethical trials of germline gene editing. N Engl J Med 377(20):1911–1913. https://doi.org/10.1056/NEJMp1711000
Mintz RL, Loike JD, Fischbach RL (2019) Will CRISPR germline engineering close the door to an open future? Sci Eng Ethics 25(5):1409–1423
Feinberg J (1980) The child’s right to an open future. In: Aiken W, LaFollette H (eds) Whose child? Rowman & Littlefield, Totowa, pp 124–153
Agar N (2019) Why we should defend gene editing as eugenics. Camb Q Healthc Ethics 28(1):9–19
Check E (2016) Tomorrow’s children. Nature 530(25):402–405. https://doi.org/10.1038/530402a
Nuffield Council on Bioethics (2018) Genome editing and human reproduction: Social and ethical issues. Nuffield Council on Bioethics, London
Hildebrandt CC, Marron JM (2018) Justice in CRISPR/Cas9 research and clinical applications. AMA J Ethics 20(9):826–833
Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Greely HT (2015) A prudent path forward for genomic engineering and germline gene modification. Science 348(6230):36–38. https://doi.org/10.1126/science.aab1028
Rosenbaum L (2019) The future of gene editing-toward scientific and social consensus. N Engl J Med 380(10):971–975
Acknowledgements
Grup Investigació en Bioètica (GIB): David Lorenzo1,4, Montse Esquerda1,5, Margarita Bofarull1, Helena Roig1, Victoria Cusí1, Francisco J. Cambra1,3, Joan Carrera1, Francesc Palau2,3
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenzo, D., Esquerda, M., Palau, F. et al. Ethics and Genomic Editing Using the Crispr-Cas9 Technique: Challenges and Conflicts. Nanoethics 16, 313–321 (2022). https://doi.org/10.1007/s11569-022-00425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11569-022-00425-y