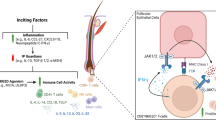
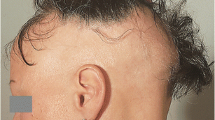
Abstract
Patients suffering from alopecia areata (AA) can lose hair in focal regions, the complete scalp, including eyelashes and eyebrows, or even the entire body. The exact pathology is not yet known, but the most described theory is a collapse of the immune privilege system, which can be found in some specific regions of the body. Different treatment options, local and systemic, are available, but none of them have been proven to be effective in the long term as well for every treatment there should be considered for the possible side effects. In many cases, treated or non-treated, relapse often occurs. The prognosis is uncertain and is negatively influenced by the subtypes alopecia totalis and alopecia universalis and characteristics such as associated nail lesions, hair loss for more than 10 years and a positive familial history. The unpredictable course of the disease also makes it a mental struggle and AA patients are more often associated with depression and anxiety compared to the healthy population. Research into immunology and genetics, more particularly in the field of dendritic cells (DC), is recommended for AA as there is evidence of the possible role of DC in the treatment of other autoimmune diseases such as multiple Sclerosis and cancer. Promising therapies for the future treatment of AA are JAK-STAT inhibitors and PRP.




Similar content being viewed by others
References
Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397–403. https://doi.org/10.2147/CCID.S53985.
Marwah M, Nadkarni N, Patil S. ’Ho-ver’ing over alopecia areata: histopathological study of 50 cases. Int J Trichol. 2014;6(1):13–8. https://doi.org/10.4103/0974-7753.136749.
Tan SP, Weller RB. Sudden whitening of the hair in an 82-year-old woman: the ‘overnight greying’ phenomenon. Clin Exp Dermatol. 2012;37(4):458–9. https://doi.org/10.1111/j.1365-2230.2011.04211.x.
Dinh QQ, Chong AH. A case of widespread non-pigmented hair regrowth in diffuse alopecia areata. Australas J Dermatol. 2007;48(4):221–3. https://doi.org/10.1111/j.1440-0960.2007.00390.x.
Tobin DJ, Fenton DA, Kendall MD. Ultrastructural observations on the hair bulb melanocytes and melanosomes in acute alopecia areata. J Invest Derm. 1990;94(6):803–7. https://doi.org/10.1111/1523-1747.ep12874660.
Finner AM. Alopecia areata: clinical presentation, diagnosis, and unusual cases. Dermatol Ther. 2011;24(3):348–54. https://doi.org/10.1111/j.1529-8019.2011.01413.x.
Yesudian P, Thambiah AS. Perinevoid alopecia. an unusual variety of alopecia areata. Arch Dermatol. 1976;112(10):1432–4.
Sato-Kawamura M, Aiba S, Tagami H. Acute diffuse and total alopecia of the female scalp .a new subtype of diffuse alopecia areata that has a favorable prognosis. Dermatology. 2002;205(4):367–73. https://doi.org/10.1159/000066435.
Rodgers AR. Why finding a treatment for alopecia areata is important: a multifaceted perspective. J Invest Derm Symp. 2018;19(1):S51–3. https://doi.org/10.1016/j.jisp.2017.10.008.
Hunt N, McHale S. The psychological impact of alopecia. BMJ (Clin Res). 2005;331(7522):951–3. https://doi.org/10.1136/bmj.331.7522.951.
Azzawi S, Penzi LR, Senna MM. Immune privilege collapse and alopecia development: is stress a factor. Skin Appendage Disord. 2018;4(4):236–44. https://doi.org/10.1159/000485080.
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines–part II. national alopecia areata foundation. J Am Acad Dermatol. 2004;51(3):440–7. https://doi.org/10.1016/j.jaad.2003.09.032.
Jang YH, Moon SY, Lee WJ, Lee SJ, Lee WK, Park BC, et al. Alopecia areata progression index, a scoring system for evaluating overall hair loss activity in alopecia areata patients with pigmented hair: a development and reliability assessment. Dermatology. 2016;232(2):143–9. https://doi.org/10.1159/000442816.
Lee H, Choe SJ, Lee WS. Method for describing patterns and distributions of alopecia areata which may be helpful for patient characterization and predicting prognosis. J Dermatol. 2019. https://doi.org/10.1111/1346-8138.14989.
Hordinsky MK. Overview of alopecia areata. J Invest Derm Symp. 2013;16(1):S13–5. https://doi.org/10.1038/jidsymp.2013.4.
Xin C, Sun X, Lu L, Yang R, Shan L, Wang Y. Increased incidence of thyroid disease in patients with alopecia areata: a systematic review and meta-analysis. Dermatology. 2019;2019:1–4. https://doi.org/10.1159/000502025.
Chelidze K, Lipner SR. Nail changes in alopecia areata: an update and review. Int J Dermatol. 2018;57(7):776–83. https://doi.org/10.1111/ijd.13866.
Majid I, Keen MA. Alopecia areata: an update. British J Med Practitioners. 2012;5(3):a530.
Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata Nat Rev Dis Primers. 2017;3:17011. https://doi.org/10.1038/nrdp.2017.11.
Kossard S. Frontal fibrosing alopecia, just lichen planopilaris? J Am Acad Dermatol. 2019;81(2):e51. https://doi.org/10.1016/j.jaad.2019.02.072.
Poblet E, Jiménez F, Pascual A, Piqué E. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45(4):375–80. https://doi.org/10.1111/j.1365-4632.2006.02507.x.
Li DG, Huang KP, Xia FD, Joyce C, Scott DA, Qureshi AA, et al. Development and pilot-testing of the alopecia areata assessment tool (ALTO). PLoS ONE. 2018;13(6):e0196517. https://doi.org/10.1371/journal.pone.0196517.
Wyrwich KW, Kitchen H, Knight S, Aldhouse NVJ, Macey J, Nunes FP, et al. Development of the scalp hair assessment patient-reported outcome (pro) measure for alopecia areata. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.19024.
Werner B, Mulinari-Brenner F. Clinical and histological challenge in the differential diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata–part II. An Bras Dermatol. 2012;87(6):884–90.
Guttikonda AS, Aruna C, Ramamurthy DV, Sridevi K, Alagappan SK. Evaluation of clinical significance of dermoscopy in alopecia areata. Indian J Dermatol. 2016;61(6):628–33. https://doi.org/10.4103/0019-5154.193668.
Mahmoudi H, Salehi M, Moghadas S, Ghandi N, Teimourpour A, Daneshpazhooh M. Dermoscopic findings in 126 patients with alopecia areata: a cross-sectional study. Int J Trichology. 2018;10(3):118–23. https://doi.org/10.4103/ijt.ijt_102_17.
Pirmez R. Revisiting coudability hairs in alopecia areata: the story behind the name. Skin Appendage Disord. 2016;2(1–2):76–8. https://doi.org/10.1159/000448811.
Waskiel A, Rakowska A, Sikora M, Olszewska M, Rudnicka L. Trichoscopy of alopecia areata: an update. J Dermatol. 2018;45(6):692–700. https://doi.org/10.1111/1346-8138.14283.
Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: diagnosis simplified. Clin Exp Dermatol. 2013;5(4):170–8. https://doi.org/10.4103/0974-7753.130385.
Whiting DA. The histopathology of alopecia areata in vertical and horizontal sections. Dermatol Ther. 2001;14(4):297–305. https://doi.org/10.1046/j.1529-8019.2001.01037.x.
Mitchell AJ, Krull EA. Alopecia areata: Pathogenesis and treatment. J Am Acad Dermatol. 1984;11(5, Part 1):763–75. https://doi.org/10.1016/S0190-9622(84)80450-8.
Ito T, Hashizume H, Shimauchi T, Funakoshi A, Ito N, Fukamizu H, et al. CXCL10 produced from hair follicles induces Th1 and Tc1 cell infiltration in the acute phase of alopecia areata followed by sustained Tc1 accumulation in the chronic phase. J Dermatol Sci. 2013;69(2):140–7. https://doi.org/10.1016/j.jdermsci.2012.12.003.
Peckham SJ, Sloan SB, Elston DM. Histologic features of alopecia areata other than peribulbar lymphocytic infiltrates. J Am Acad Dermatol. 2011;65(3):615–20. https://doi.org/10.1016/j.jaad.2011.02.017.
Elston DM, McCollough ML, Bergfeld WF, Liranzo MO, Heibel M. Eosinophils in fibrous tracts and near hair bulbs: a helpful diagnostic feature of alopecia areata. J Am Acad Dermatol. 1997;37(1):101–6. https://doi.org/10.1016/s0190-9622(97)70219-6.
Happle R, Klein HM, Macher E. Topical immunotherapy changes the composition of the peribulbar infiltrate in alopecia areata. Arch Dermatol Res. 1986;278(3):214–8. https://doi.org/10.1007/bf00412926.
Whiting DA. Histopathology of alopecia areata in horizontal sections of scalp biopsies. J Invest Dermatol. 1995;104(5 Suppl):26s-s27. https://doi.org/10.1038/jid.1995.46.
Fuentes-Duculan J, Gulati N, Bonifacio KM, Kunjravia N, Zheng X, Suarez-Farinas M, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25(4):282–6. https://doi.org/10.1111/exd.12918.
Kennedy Crispin M, Ko JM, Craiglow BG, Li S, Shankar G, Urban JR, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. https://doi.org/10.1172/jci.insight.89776.
Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–87. https://doi.org/10.1016/j.jaci.2015.06.032.
Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139(12):1555–9. https://doi.org/10.1001/archderm.139.12.1555.
Singh K, Sharma S, Singh UR, Bhattacharya SN. A Comparison of vertical and transverse sections in the histological diagnosis of alopecia areata scalp biopsy specimens. Int J Trichol. 2016;8(3):111–5. https://doi.org/10.4103/0974-7753.188964.
Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18(4):577–84. https://doi.org/10.1007/s10238-018-0511-8.
Marahatta S, Agrawal S, Khan S. Study on serum vitamin D in alopecia areata patients. J Nepal Health Res Counc. 2019;17(1):21–5. https://doi.org/10.33314/jnhrc.1475.
Unal M, Gonulalan G. Serum vitamin D level is related to disease severity in pediatric alopecia areata. J Cosmet Dermatol. 2018;17(1):101–4. https://doi.org/10.1111/jocd.12352.
Rehman F, Dogra N, Wani MA. Serum vitamin d levels and alopecia areata- a hospital based case-control study from North-India. Int J Trichology. 2019;11(2):49–57. https://doi.org/10.4103/ijt.ijt_3_19.
Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1214–21. https://doi.org/10.1111/jdv.14987.
Thompson JM, Mirza MA, Park MK, Qureshi AA, Cho E. The role of micronutrients in alopecia areata: a review. Am J Clin Dermatol. 2017;18(5):663–79. https://doi.org/10.1007/s40257-017-0285-x.
Brzezinska-Wcislo L, Bergler-Czop B, Wcislo-Dziadecka D, Lis-Swiety A. New aspects of the treatment of alopecia areata. Postepy Dermatol Alergol. 2014;31(4):262–5. https://doi.org/10.5114/pdia.2014.40923.
Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24(3):355–63. https://doi.org/10.1111/j.1529-8019.2011.01419.x.
Burroway B, Griggs J, Tosti A. Alopecia totalis and universalis long-term outcomes: a review. J Eur Acad Dermatol Venereol. 2019. https://doi.org/10.1111/jdv.15994.
Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol. 1998;139(5):846–50. https://doi.org/10.1046/j.1365-2133.1998.02511.x.
Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–7. https://doi.org/10.1038/nature09114.
Cools N, Petrizzo A, Smits E, Buonaguro FM, Tornesello ML, Berneman Z, et al. Dendritic cells in the pathogenesis and treatment of human diseases: a janus bifrons? Immunotherapy. 2011;3(10):1203–22. https://doi.org/10.2217/imt.11.110.
Hoffmann JM, Schmitt M, Ni M, Schmitt A. Next-generation dendritic cell-based vaccines for leukemia patients. Immunotherapy. 2017;9(2):173–81. https://doi.org/10.2217/imt-2016-0116.
Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Invest Derm Symp P. 2003;8(2):188–94. https://doi.org/10.1046/j.1087-0024.2003.00807.x.
Fenster RJ, Eisen JL. Checking the brain’s immune privilege: evolving theories of brain-immune interactions. Biol Psychiat. 2017;81(2):e7–9. https://doi.org/10.1016/j.biopsych.2016.10.027.
Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7(4):354–9. https://doi.org/10.1038/ni1328.
Kinori M, Bertolini M, Funk W, Samuelov L, Meyer KC, Emelianov VU, et al. Calcitonin gene-related peptide (CGRP) may award relative protection from interferon-gamma-induced collapse of human hair follicle immune privilege. Exp Dermatol. 2012;21(3):223–6. https://doi.org/10.1111/j.1600-0625.2011.01432.x.
Breitkopf T, Lo BKK, Leung G, Wang E, Yu M, Carr N, et al. Somatostatin expression in human hair follicles and its potential role in immune privilege. J Invest Derm. 2013;133(7):1722–30. https://doi.org/10.1038/jid.2013.53.
Schmid P, Itin P, Rufli T. In situ analysis of transforming growth factors-beta (TGF-beta 1, TGF-beta 2, TGF-beta 3) and TGF-beta type II receptor expression in basal cell carcinomas. Br J Dermatol. 1996;134(6):1044–51.
Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122(6):658–66. https://doi.org/10.5555/uri:pii:002221439390247V.
Kang H, Wu WY, Lo BK, Yu M, Leung G, Shapiro J, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2010;130(11):2677–80. https://doi.org/10.1038/jid.2010.180.
Gilhar A. Collapse of immune privilege in alopecia areata: coincidental or substantial? J Invest Dermatol. 2010;130(11):2535–7. https://doi.org/10.1038/jid.2010.260.
Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66(6):541–54.
Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–37.
Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol. 2013;22(12):785–9. https://doi.org/10.1111/exd.12264.
Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. American J Pathol. 2004;164(2):623–34. https://doi.org/10.1016/s0002-9440(10)63151-3.
Ruckert R, Hofmann U, van der Veen C, Bulfone-Paus S, Paus R. MHC class I expression in murine skin: developmentally controlled and strikingly restricted intraepithelial expression during hair follicle morphogenesis and cycling, and response to cytokine treatment in vivo. J Invest Dermatol. 1998;111(1):25–30. https://doi.org/10.1046/j.1523-1747.1998.00228.x.
Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol. 2014;14(6):377–91. https://doi.org/10.1038/nri3667.
Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. https://doi.org/10.1146/annurev.immunol.23.021704.115601.
Chan AY, Anderson MS. Central tolerance to self revealed by the autoimmune regulator. Ann N Y Acad Sci. 2015;1356:80–9. https://doi.org/10.1111/nyas.12960.
Bruserud O, Oftedal BE, Wolff AB, Husebye ES. AIRE-mutations and autoimmune disease. Curr Opin Immunol. 2016;43:8–15. https://doi.org/10.1016/j.coi.2016.07.003.
Tazi-Ahnini R, Cork MJ, Gawkrodger DJ, Birch MP, Wengraf D, McDonagh AJ, et al. Role of the autoimmune regulator (AIRE) gene in alopecia areata: strong association of a potentially functional AIRE polymorphism with alopecia universalis. Tissue Antigens. 2002;60(6):489–95. https://doi.org/10.1034/j.1399-0039.2002.600604.x.
Chung CY, Ysebaert D, Berneman ZN, Cools N. Dendritic cells: cellular mediators for immunological tolerance. Clin Dev Immunol. 2013;2013:972865. https://doi.org/10.1155/2013/972865.
Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–69. https://doi.org/10.1038/nri3254.
Volpe E, Sambucci M, Battistini L, Borsellino G. Fas-fas ligand: checkpoint of T cell functions in multiple sclerosis. Front Immunol. 2016;7:382. https://doi.org/10.3389/fimmu.2016.00382.
Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The Importance of dendritic cells in maintaining immune tolerance. J Immunol. 2017;198(6):2223–31. https://doi.org/10.4049/jimmunol.1601629.
Yanofsky VR, Mitsui H, Felsen D, Carucci JA. Understanding dendritic cells and their role in cutaneous carcinoma and cancer immunotherapy. Clin Dev Immunol. 2013;2013:624123. https://doi.org/10.1155/2013/624123.
Jaitley S, Saraswathi TR. Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol: JOMFP. 2012;16(2):239–44. https://doi.org/10.4103/0973-029X.99077.
Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140(1):22–30. https://doi.org/10.1111/imm.12117.
Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234(1):120–41. https://doi.org/10.1111/j.0105-2896.2009.00886.x.
Ayala-Garcia I, Hernandez-Segura AM, Castell-Rodriguez A, Alvarez Perez SJ, Tellez BH, Ramirez-Gonzalez MD. Participation of epidermal langerhans cells in human pathology and their potential as targets for drug development: a review of literature. Proc West Pharmacol Soc. 2005;48:13–20.
Upadhyay J, Upadhyay RB, Agrawal P, Jaitley S, Shekhar R. Langerhans cells and their role in oral mucosal diseases. N Am J Med Sci. 2013;5(9):505–14. https://doi.org/10.4103/1947-2714.118923.
Bangert C, Brunner PM, Stingl G. Immune functions of the skin. Clin Dermatol. 2011;29(4):360–76. https://doi.org/10.1016/j.clindermatol.2011.01.006.
Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37(1):60–73. https://doi.org/10.1016/j.immuni.2012.04.012.
Takayama K, Yokozeki H, Ghoreishi M, Satoh T, Umeda T, Nishioka K, et al. IL-4 Inhibits the migration of human langerhans cells through the downregulation of TNF receptor II expression. J Invest Derm. 1999;113(4):541–6. https://doi.org/10.1046/j.1523-1747.1999.00629.x.
Brinkman CC, Rouhani SJ, Srinivasan N, Engelhard VH. Peripheral tissue homing receptors enable T cell entry into lymph nodes and affect the anatomical distribution of memory cells. J Immunol. 2013;191(5):2412–25. https://doi.org/10.4049/jimmunol.1300651.
Gupta P, Freyschmidt-Paul P, Vitacolonna M, Kiessling S, Hummel S, Hildebrand D, et al. A chronic contact eczema impedes migration of antigen-presenting cells in alopecia areata. J Invest Dermatol. 2006;126(7):1559–73. https://doi.org/10.1038/sj.jid.5700328.
Toebak MJ, Gibbs S, Bruynzeel DP, Scheper RJ, Rustemeyer T. Dendritic cells: biology of the skin. Contact Dermat. 2009;60(1):2–20. https://doi.org/10.1111/j.1600-0536.2008.01443.x.
De Koker SLB, Willart MA, van Kooyk Y, Grooten J, Vervaet C, Remona JP, De Geest B. Designing polymeric particles for antigen delivery. Chem Soc Rev. 2011;40:320–39. https://doi.org/10.1039/B914943K.
Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19(1):3–19. https://doi.org/10.1016/j.cytogfr.2007.10.006.
Abou Rahal J, Kurban M, Kibbi AG, Abbas O. Plasmacytoid dendritic cells in alopecia areata: missing link? J Eur Acad Dermatol Venereol. 2016;30(1):119–23. https://doi.org/10.1111/jdv.12932.
Suárez-Fariñas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–87. https://doi.org/10.1016/j.jaci.2015.06.032.
Herrmann M, Scholmerich J, Straub RH. Stress and rheumatic diseases. Rheum Dis Clin North America. 2000;26(4):737–63.
Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9(5):A271–6. https://doi.org/10.1016/j.autrev.2009.11.014.
Misery L, Rousset H. Is alopecia areata a psychosomatic disease? La Revue de medecine interne. 2001;22(3):274–9.
Ghanizadeh A, Ayoobzadehshirazi A. A review of psychiatric disorders comorbidities in patients with alopecia areata. Int J Trichol. 2014;6(1):2–4. https://doi.org/10.4103/0974-7753.136746.
Titeca G, Goudetsidis L, Francq B, Sampogna F, Gieler U, Tomas-Aragones L, et al. “The psychosocial burden of alopecia areata and androgenetica”: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Eur Acad Dermatol Venereol. 2019. https://doi.org/10.1111/jdv.15927.
Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case-control study of adverse events in a national database. J Drugs Dermatol : JDD. 2015;14(7):681–4.
Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34(7):1225–31. https://doi.org/10.1007/s10067-014-2846-1.
Chu CH, Cheng YP, Chan JY. Alopecia areata after vaccination: recurrence with rechallenge. Pediatr Dermatol. 2016;33(3):e218–9. https://doi.org/10.1111/pde.12849.
Hammerschmidt M, Mulinari BF. Efficacy and safety of methotrexate in alopecia areata. An Bras Dermatol. 2014;89(5):729–34.
Horst HJ, Flad HD. Corticosteroid-interleukin 2 interactions: inhibition of binding of interleukin 2 to interleukin 2 receptors. Clin Exp Immunol. 1987;68(1):156–61.
Lee GC, Yang IM, Kim BJ, Woo JT, Kim SW, Kim JW, et al. Identification of glucocorticoid response element of the rat TRH gene. Korean J Intern Med. 1996;11(2):138–44. https://doi.org/10.3904/kjim.1996.11.2.138.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24. https://doi.org/10.1038/nri.2017.142.
Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;2002(138):re9. https://doi.org/10.1126/stke.2002.138.re9.
Alsantali A. Alopecia areata: a new treatment plan. Clin Cosmet Investig Dermatol. 2011;4:107–15. https://doi.org/10.2147/ccid.S22767.
Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 005% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49(1):96–8. https://doi.org/10.1067/mjd.2003.423.
Kalkoff KW, Macher E. Growing of hair in alopecia areata and maligna after intracutaneous hydrocortisone injection. Hautarzt. 1958;9(10):441–51.
Kumaresan M. Intralesional steroids for alopecia areata. Int J Trichology. 2010;2(1):63–5. https://doi.org/10.4103/0974-7753.66920.
Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145(3):385–405.
Wilkerson MG, Henkin J, Wilkin JK, Smith RG. Squaric acid and esters: analysis for contaminants and stability in solvents. J Am Acad Dermatol. 1985;13(2 Pt 1):229–34.
Singh G, Lavanya MS. Topical Immunotherapy in alopecia areata. Int J Trichol. 2010;2(1):36–9. https://doi.org/10.4103/0974-7753.66911.
Gong Y, Zhao Y, Zhang X, Qi S, Li S, Ye Y, et al. Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp Dermatol. 2020;29(3):231–8. https://doi.org/10.1111/exd.13758.
Cotellessa C, Peris K, Caracciolo E, Mordenti C, Chimenti S. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol. 2001;44(1):73–6. https://doi.org/10.1067/mjd.2001.109309.
Aghaei S. Topical immunotherapy of severe alopecia areata with diphenylcyclopropenone (DPCP): experience in an Iranian population. BMC Dermatol. 2005;5(1):6. https://doi.org/10.1186/1471-5945-5-6.
Chiang KS, Mesinkovska NA, Piliang MP, Bergfeld WF. Clinical efficacy of diphenylcyclopropenone in alopecia areata: retrospective data analysis of 50 patients. J Invest Derm Symp P. 2015;17(2):50–5. https://doi.org/10.1038/jidsymp.2015.28.
Weise K, Kretzschmar L, John SM, Hamm H. Topical immunotherapy in alopecia areata: anamnestic and clinical criteria of prognostic significance. Dermatology. 1996;192(2):129–33. https://doi.org/10.1159/000246337.
Jang YH, Jung HJ, Moon SY, Lee WJ, Lee SJ, Lee WK, et al. Systematic review and quality analysis of studies on the efficacy of topical diphenylcyclopropenone treatment for alopecia areata. J Am Acad Dermatol. 2017;77(1):170–2. https://doi.org/10.1016/j.jaad.2017.03.015.
Goren A, McCoy J, Kovacevic M, Situm M, Chitalia J, Dhurat R, et al. The effect of topical minoxidil treatment on follicular sulfotransferase enzymatic activity. J Biol Regul Homeost Agents. 2018;32(4):937–40.
Goren A, Shapiro J, Roberts J, McCoy J, Desai N, Zarrab Z, et al. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. 2015;28(1):13–6. https://doi.org/10.1111/dth.12164.
Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–94. https://doi.org/10.1111/j.1365-2133.2004.05785.x.
Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AG, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50(4):541–53. https://doi.org/10.1016/j.jaad.2003.06.014.
Ghonemy S, Alarawi A, Bessar H. Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. J Dermatol Treat. 2019;47:1–6. https://doi.org/10.1080/09546634.2019.1654070.
Goren A, Sharma A, Dhurat R, Shapiro J, Sinclair R, Situm M, et al. Low-dose daily aspirin reduces topical minoxidil efficacy in androgenetic alopecia patients. Dermatol Ther. 2018;31(6):e12741. https://doi.org/10.1111/dth.12741.
Gonzalez M, Landa N, Gardeazabal J, Calderon MJ, Bilbao I, Diaz Perez JL. Generalized hypertrichosis after treatment with topical minoxidil. Clin Exp Dermatol. 1994;19(2):157–8. https://doi.org/10.1111/j.1365-2230.1994.tb01147.x.
Peluso AM, Misciali C, Vincenzi C, Tosti A. Diffuse hypertrichosis during treatment with 5% topical minoxidil. Br J Dermatol. 1997;136(1):118–20.
Dawber RP, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J Eur Acad Dermatol Venereol. 2003;17(3):271–5.
Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Dis. 2012;6(2):130–6.
Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136-41.e5. https://doi.org/10.1016/j.jaad.2017.02.054.
Sung CT, Juhasz ML, Choi FD, Mesinkovska NA. The Efficacy of topical minoxidil for non-scarring alopecia: a systematic review. J Drugs Dermatol: JDD. 2019;18(2):155–60.
Situm M, Bulat V, Majcen K, Dzapo A, Jezovita J. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Collegium Antropologicum. 2014;38(4):1249–53.
Passeron T, Ortonne JP. Use of the 308-nm excimer laser for psoriasis and vitiligo. Clin Dermatol. 2006;24(1):33–42. https://doi.org/10.1016/j.clindermatol.2005.10.024.
Sigmundsdottir H, Johnston A, Gudjonsson JE, Valdimarsson H. Narrowband-UVB irradiation decreases the production of pro-inflammatory cytokines by stimulated T cells. Arch Dermatol Res. 2005;297(1):39–42. https://doi.org/10.1007/s00403-005-0565-9.
Byun JW, Moon JH, Bang CY, Shin J, Choi GS. Effectiveness of 308-nm excimer laser therapy in treating alopecia areata, determined by examining the treated sides of selected alopecic patches. Dermatology. 2015;231(1):70–6. https://doi.org/10.1159/000381912.
Welsh O. Phototherapy for alopecia areata. Clin Dermatol. 2016;34(5):628–32. https://doi.org/10.1016/j.clindermatol.2016.05.014.
Mohamed Z, Bhouri A, Jallouli A, Fazaa B, Kamoun M, Mokhtar I. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J Eur Acad Dermatol Venereol. 2005;19(5):552–5. https://doi.org/10.1111/j.1468-3083.2005.01226.x.
Majumdar B, De A, Ghosh S, Sil A, Sarda A, Lahiri K, et al. “Turban PUVAsol:” a simple, novel, effective, and safe treatment option for advanced and refractory cases of alopecia areata. Int J Trichol. 2018;10(3):124–8. https://doi.org/10.4103/ijt.ijt_95_17.
Mitchell AJ, Douglass MC. Topical photochemotherapy for alopecia areata. J Am Acad Dermatol. 1985;12(4):644–9. https://doi.org/10.1016/S0190-9622(85)70088-6.
Abdullah An, Keczkes K. Cutaneous and ocular side-effects of PUVA photochemotherapy-a 10-year follow-up study. Clin Exp Dermatol. 1984;14(6):421–6. https://doi.org/10.1111/j.1365-2230.1989.tb02602.x.
Burton JL, Shuster S. Large doses of glucocorticoid in the treatment of alopecia areata. Acta dermato-venereologica. 1975;55(6):493–6.
Shreberk-Hassidim R, Ramot Y, Gilula Z, Zlotogorski A. A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol. 2016;74(2):372–4. https://doi.org/10.1016/j.jaad.2015.09.045.
Nakajima T, Inui S, Itami S. Pulse corticosteroid therapy for alopecia areata: study of 139 patients. Dermatology. 2007;215(4):320–4. https://doi.org/10.1159/000107626.
Lim S-K, Lim C-A, Kwon IS, Im M, Seo Y-J, Kim C-D, et al. Low-dose systemic methotrexate therapy for recalcitrant alopecia areata. Ann Dermatol. 2017;29(3):263–7. https://doi.org/10.5021/ad.2017.29.3.263.
Joly P. The use of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia totalis or universalis. J Am Acad Dermatol. 2006;55(4):632–6. https://doi.org/10.1016/j.jaad.2005.09.010.
Chartaux E, Joly P. Long-term follow-up of the efficacy of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia areata totalis or universalis. Ann Dermatol Venereol. 2010;137(8–9):507–13. https://doi.org/10.1016/j.annder.2010.06.031.
Rashidi T, Mahd AA. Treatment of persistent alopecia areata with sulfasalazine. Int J Dermatol. 2008;47(8):850–2. https://doi.org/10.1111/j.1365-4632.2008.03700.x.
Aghaei S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J Dermatol Venereol Leprol. 2008;74(6):611–3.
Acikgoz G, Caliskan E, Tunca M, Yeniay Y, Akar A. The effect of oral cyclosporine in the treatment of severe alopecia areata. Cutan Ocul Toxicol. 2014;33(3):247–52. https://doi.org/10.3109/15569527.2013.839997.
Lai VWY, Chen G, Gin D, Sinclair R. Cyclosporine for moderate-to-severe alopecia areata: a double-blind, randomized, placebo-controlled clinical trial of efficacy and safety. J Am Acad Dermatol. 2019;81(3):694–701. https://doi.org/10.1016/j.jaad.2019.04.053.
Sakkas LI, Mavropoulos A, Bogdanos DP. Phosphodiesterase 4 inhibitors in immune-mediated diseases: mode of action, clinical applications, current and future perspectives. Curr Med Chem. 2017;24(28):3054–67. https://doi.org/10.2174/0929867324666170530093902.
Gilhar A, Keren A, Shemer A, Ullmann Y, Paus R. Blocking potassium channels (Kv1.3): a new treatment option for alopecia areata? J Invest Derm. 2013;133(8):2088–91. https://doi.org/10.1038/jid.2013.141.
Gilhar A, Keren A, Shemer A, d’Ovidio R, Ullmann Y, Paus R. Autoimmune disease induction in a healthy human organ: a humanized mouse model of alopecia areata. J Invest Derm. 2013;133(3):844–7. https://doi.org/10.1038/jid.2012.365.
Keren A, Shemer A, Ullmann Y, Paus R, Gilhar A. The PDE4 inhibitor, apremilast, suppresses experimentally induced alopecia areata in human skin in vivo. J Dermatol Sci. 2014;77:74–6. https://doi.org/10.1016/j.jdermsci.2014.11.009.
Magdaleno-Tapial J, Valenzuela-Oñate C, Sánchez-Carazo JL, Alegre-de MV. Improvement of alopecia areata with apremilast. Australas J Dermatol. 2019;60(2):144–5. https://doi.org/10.1111/ajd.12934.
Mikhaylov D, Pavel A, Yao C, Kimmel G, Nia J, Hashim P, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29–36. https://doi.org/10.1007/s00403-018-1876-y.
Estebanez A, Estebanez N, Martin JM, Montesinos E. Apremilast in refractory alopecia areata. Int J Trichol. 2019;11(5):213–5. https://doi.org/10.4103/ijt.ijt_59_19.
Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12(1):25–36. https://doi.org/10.1038/nrrheum.2015.167.
O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. https://doi.org/10.1146/annurev-med-051113-024537.
Vincenti F, Tedesco Silva H, Busque S, O’Connell P, Friedewald J, Cibrik D, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. American J Transplant: Official J American Soc Transplant American Soc Transplant Surg. 2012;12(9):2446–56. https://doi.org/10.1111/j.1600-6143.2012.04127.x.
Liew SH, Nichols KK, Klamerus KJ, Li JZ, Zhang M, Foulks GN. Tofacitinib (CP-690,550), a Janus kinase inhibitor for dry eye disease: results from a phase 1/2 trial. Ophthalmology. 2012;119(7):1328–35. https://doi.org/10.1016/j.ophtha.2012.01.028.
Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167(3):668–77. https://doi.org/10.1111/j.1365-2133.2012.11168.x.
Strober B, Buonanno M, Clark JD, Kawabata T, Tan H, Wolk R, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169(5):992–9. https://doi.org/10.1111/bjd.12517.
Ports WC, Khan S, Lan S, Lamba M, Bolduc C, Bissonnette R, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169(1):137–45. https://doi.org/10.1111/bjd.12266.
Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2014;28(2):192–203. https://doi.org/10.1111/jdv.12081.
Menter A, Papp KA, Tan H, Tyring S, Wolk R, Buonanno M. Efficacy of tofacitinib, an oral janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol: JDD. 2014;13(3):252–6.
Gilhar A, Schrum AG, Etzioni A, Waldmann H, Paus R. Alopecia areata: animal models illuminate autoimmune pathogenesis and novel immunotherapeutic strategies. Autoimmun Rev. 2016;15(7):726–35. https://doi.org/10.1016/j.autrev.2016.03.008.
Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–9. https://doi.org/10.1038/nm.3645.
Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134(12):2988–90. https://doi.org/10.1038/jid.2014.260.
Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–8. https://doi.org/10.1016/j.jaad.2016.09.007.
Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76(1):29–32. https://doi.org/10.1016/j.jaad.2016.09.006.
Almutairi N, Nour TM, Hussain NH. Janus kinase inhibitors for the treatment of severe alopecia areata: an open-label comparative study. Dermatology. 2019;235(2):130–6. https://doi.org/10.1159/000494613.
Emer J. Platelet-rich plasma (PRP): current applications in dermatology. Skin Ther Lett. 2019;24(5):1–6.
Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg : Off J American Assoc Oral Maxillofac Surg. 2000;58(3):297–300. https://doi.org/10.1016/s0278-2391(00)90058-2.
Singh B, Goldberg LJ. Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol. 2016;17(4):359–67. https://doi.org/10.1007/s40257-016-0196-2.
Fan Y, Perez K, Dym H. Clinical uses of platelet-rich fibrin in oral and maxillofacial surgery. Dent Clin North America. 2020;64(2):291–303. https://doi.org/10.1016/j.cden.2019.12.012.
Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4(11):1317–23. https://doi.org/10.5966/sctm.2015-0107.
Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7(2):107–10. https://doi.org/10.4103/0974-2077.138352.
Khademi F, Tehranchinia Z, Abdollahimajd F, Younespour S, Kazemi-Bajestani SMR, Taheri K. The effect of platelet rich plasma on hair regrowth in patients with alopecia areata totalis: a clinical pilot study. Dermatol Ther. 2019;32(4):e12989. https://doi.org/10.1111/dth.12989.
Badran KW, Sand JP. Platelet-rich plasma for hair loss: review of methods and results. Fac Plast Surg Clin North America. 2018;26(4):469–85. https://doi.org/10.1016/j.fsc.2018.06.008.
Schippinger G, Pruller F, Divjak M, Mahla E, Fankhauser F, Rackemann S, et al. Autologous platelet-rich plasma preparations: influence of nonsteroidal anti-inflammatory drugs on platelet function. Orthop J Sports Med. 2015;3(6):2325967115588896. https://doi.org/10.1177/2325967115588896.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sterkens, A., Lambert, J. & Bervoets, A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med 21, 215–230 (2021). https://doi.org/10.1007/s10238-020-00673-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00673-w