Abstract
Objectives
The aim of this study is to analyse the trends in technology appraisals for non-small cell lung cancer (NSCLC) treatments performed by the National Institute for Health and Care Excellence (NICE) over the last ten years.
Methods
A systematic search was conducted for single technology appraisals of NSCLC drugs in the online NICE database from 2012 to 2022. Search terms used were ‘non small cell lung cancer’, and ‘NSCLC’. Appraisals that were under development or terminated as well as multiple technology appraisals were considered out of scope.
Results
In the 30 included appraisals for targeted therapies and immunotherapies within NSCLC, a total of 53 different comparators were included by NICE for 41 assorted indications or subgroups. Partitioned survival models were most frequently used, often including three health states and time horizons of up to 30 years. Throughout the decade the use of indirect comparisons was high and became more established and complex over time. Of all appraisals, 90% positively recommended the treatment for use in the UK.
Conclusion
Technology appraisals became more complex over time due to the emergence of targeted therapies and immunotherapies, leading to multiple different indications, subpopulations and comparators that needed to be included in appraisals. Partitioned Survival Analysis (PartSA) models became the cornerstone within NSCLC, with time horizons up to 30 years and over time methods for indirect treatment comparisons became more established. The majority of the appraisals resulted in a positive recommendation for reimbursement.
Key points
• Lung cancer is the leading cause of cancer mortality, with about 80% of lung cancer patients diagnosed with non-small cell lung cancer (NSCLC). The objective of this study was to analyse the trends in National Institute for Health and Care Excellence (NICE) technology appraisals for NSCLC drugs in the United Kingdom over the last decade.
• Our study showed that Partitioned Survival Analysis (PartSA) models with the use of indirect treatment comparisons are currently the cornerstone within NSCLC, with included time horizons up to 30 years. Due to the emergence of targeted therapies and immunotherapies over the last decade, fast pace of updates in standard of care within NSCLC were seen. This has led to the required inclusion of multiple indications, subpopulations and comparators into the appraisals, resulting in an increased level of complexity.
• Technology appraisals in NSCLC will become even more intricate with an increased importance for more guidance on the elaboration of indirect comparisons, as new mutations and expressions are still being identified and innovative therapies are emerging, such as treatments for the mutations or expressions BRAF, HER2, HER3, KRAS, MET, and pan-tumour molecular targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and objective
Lung cancer is the leading cause of cancer mortality, with about 80% of lung cancer patients diagnosed with non-small cell lung cancer (NSCLC) [1, 2]. Next to a high mortality rate, lung cancer is associated with high morbidity and healthcare costs [1]. Over the last decade, systemic anticancer therapy for advanced or metastatic NSCLC evolved to more personalized therapy based on tumour histology and oncogene biomarker status. In this period, new NSCLC treatments emerged with focusing more on specific mutations or expressions like anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), c-ros oncogene 1 (ROS1) and programmed death-(ligand) 1 (PD-(L)1) expression. These emerging innovative therapies are leading to better patient outcomes such as improved overall survival, but also come with a significant burden on healthcare budgets [3].
Globally, governments need to allocate healthcare interventions within limited healthcare budgets. Economic evaluations are increasingly being conducted to estimate a value-based cost-effective (negotiated) price for treatments. In the UK, the cost-effectiveness of interventions is of crucial importance in the decision-making process by the National Health Service (NHS), which is informed by guidance and recommendations of the National Institute for Health and Care Excellence (NICE) [4]. NICE is one of the global leaders in performing health technology appraisals. Since July 2016, all new cancer drugs are considered for recommendation into the Cancer Drugs Fund (CDF) when there is significant remaining clinical uncertainty as well as the related uncertainty about the cost-effectiveness [5]. After the period in the CDF, the treatment will be re-evaluated for recommendation in the NHS. To evaluate an intervention with regards to the cost-effectiveness, NICE considers it most appropriate to use an acceptable range for the incremental cost-effectiveness ratio (ICER) as a willingness-to-pay (WTP) threshold. Judgement on the WTP threshold above £20,000 per quality-adjusted life year (QALY) considers a combination of the following factors: degree of certainty around the ICER, the way the change in health-related quality of life (HRQoL) is captured, the innovative nature of the technology, meeting end-of-life criteria, and non-health related aspects [6]. When a treatment is considered life-extending at the end of life, usually the NICE applies a WTP threshold of £50,000 per QALY. Two key criteria need to be met for this consideration: (1) The treatment is indicated for patients with a short life expectancy (< 24 months), (2) There is sufficient evidence to indicate that the treatment’s prospect has a mean value of three months additional extension to life in comparison to the current NHS treatment [6]. The assumption is that when treatments for new mutations become available, NICE will first consider a WTP threshold of £50,000 per QALY. When more treatments for the same mutation will come available to the market, the WTP threshold for first line treatment will decrease to £20,000–30,000 per QALY and for second line treatment this will then become £50,000 per QALY.
Over the last decade various reviews of economic evaluations for interventions in oncology and in NSCLC have been published. Multiple studies analysed how survival was modelled for cancer treatments in NICE appraisals [7,8,9,10]. These studies were focused on appraisals performed by NICE in oncology although these were not focused on treatments within NSCLC. When considering reviews that focused on NSCLC, these reviews did not consider appraisals performed by NICE, but analysed cost-effectiveness of targeted mutations, such as EGFR and ALK, [11, 12] or immune checkpoint inhibitors within NSCLC [13, 14]. Another study focused on economic evaluations in second and later lines of therapy in NSCLC, but this study only included first generation tyrosine kinase inhibitors (TKIs) and pemetrexed, which is limited in the context of all emerging medicine developments in the field of NSCLC [15]. Also, this study reported lifetime time horizons that only concerned two to three years, which was considered similar as the terminal nature of the disease and the high mortality rates [15]. Current technology appraisals in the UK usually consider a time horizon between 10 and 30 years depending on mutation or expression, which is also in line with the major clinical developments in the treatment of NSCLC seen over the last ten years.
It is to be expected that due to the above-mentioned dynamic landscape, the economic evaluations of treatments of NSCLC changed over the years. Analysing NICE technology appraisals of NSCLC-treatments over the last ten years potentially uncovers relevant trends. Therefore, the objective of this study is to analyse the trends in NICE technology appraisals for NSCLC drugs in the last decade. This study focuses on the indications within NSCLC, model specifics and outcomes, indirect treatment comparisons, and recommendations. The outcomes of our study potentially inform the development and market access process of new oncology therapies with regards to what steps need to be considered both from a clinical outcomes research perspective as well as a health economics perspective.
Methods
Study design
The website of NICE contains a transparent database for all technology appraisals and other guidance. This review focused on Single Technology Appraisals (STAs), designed to appraise an intervention for a single indication, for therapies of NSCLC over the last decade.
A systematic search was conducted on January 27, 2022, for published STAs in the online database of NICE in the UK (nice.org.uk) between the period: 1 January 2012 and 1 January 2022. The search terms used concerned ‘non small cell lung cancer’, and ‘NSCLC’. The applied filters concerned Source – ‘NICE’, and Evidence type – ‘Guidance and Policy’ and ‘Prescribing and Technical information’. Terminated appraisals, appraisals in development and Multiple Technology Appraisals (MTAs) were out of scope of this study. All appraisals were screened independently by LW and SW. Any disagreements were resolved by consensus.
Data extraction
Data from each technology appraisal was extracted from the most recent committee papers and committee slides from NICE. When specific data was not found in the most recent guidance due to redacted text and data (black boxes) or references to earlier appraisal rounds, additional data was extracted from committee papers of former appraisal rounds when available. Data from budget impact analyses, which are a standard part of the technology appraisal, were predominantly not publicly available and were therefore out of scope.
Data items
A data extraction form was created to corroborate the needed information to meet our study objective. Extracted data included disease area and indication (generic name, mutation/expression, line of treatment), overview of technology appraisal details (publication date, recommendations, uptake in CDF), economic model characteristics (model structure, time horizon, cycle length), cost-effectiveness outcomes and WTP thresholds, uncertainty analyses, patient reported outcome measures, comparators, and indirect treatment comparisons. See appendix A for further details of the extraction form.
Outcomes
This review aimed to identify timely trends due to the clinical developments in NSCLC with regards to overall trends in the treatment landscape, recommendations by NICE, cost-effectiveness models used and the inclusion of relevant comparators for (in)direct treatment comparisons, and possible trends in patient reported outcomes measures.
Results
Screening process
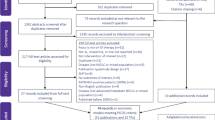
The initial search yielded 119 records, of which 55 were excluded due to the following reasons: other indication focus (N = 25), appraising a non-drug medical technology (N = 7), concerned disease management guidance (N = 13), information update (N = 1), concerned a multiple technology appraisal (N = 1), and appraisal was terminated (N = 8). Also, records were excluded as these were updated and replaced by other TA numbers, and deduplication of the resulting 60 records excluded another 30 records. Finally, this resulted in a total of 30 technology appraisals for treatments in NSCLC that were included for the data extraction.
Data extraction
The included appraisals concerned eleven indications including PD-L1 expression, eight for ALK mutations, six for EGFR mutations, two for the ROS1 oncogene, one for NSCLC of adenocarcinoma histology (nintedanib), one for previously treated NSCLC (ramucirumab), and one for maintenance treatment for NSCLC (pemetrexed).
Among these technology appraisals, 17 concerned first line indications. One of the 17 appraisals concerned an indication for first and later lines (crizotinib TA529) and another appraisal (atezolizumab TA584) included both first line treatment for PD-L1 expression of 0–49% and as well as later line when targeted therapy for EGFR-positive or ALK-positive NSCLC failed. In addition, 12 appraisals concerned second line indications of which two appraisals indicated second and later lines (pembrolizumab TA428, and lorlatinib TA628). Lastly, one technology appraisal for pemetrexed concerned maintenance treatment.
In Fig. 1 and in more detail in Table 1 an overview is provided of the extracted NICE technology appraisals 2012–2022 in chronological order of publication year. Trends will be further discussed per mutation or expression with a focus on mutations EGFR, ALK and ROS1, and on PD-(L)1 expression.
Trends in NSCLC treatments landscape in technology appraisals
EGFR mutation positive NSCLC
EGFR TKIs were one of the first molecular targeted therapies in NSCLC and replaced platinum-based combination chemotherapy as first line therapy for patients with EGFR-positive NSCLC. These treatments emerged rapidly in one decade with three generations of TKIs succeeding one another. Technology appraisals of the EGFR TKIs are mainly indicated for first line indications (see Fig. 1). With the two appraisals of the third generation TKI osimertinib in 2020, a new trend for the EGFR TKIs seems to be introduced in which first the second line indication will be appraised, and the first line appraisal will follow consecutively.
ALK- and ROS1 positive mutations in NSCLC
Improved understanding of oncogenes like ALK led the TKI crizotinib to be the first treatment appraised and recommended drug for NSCLC with ALK gene rearrangements in the UK. After the first appraisal of crizotinib for first line treatment of ALK-positive NSCLC, four other TKIs (ceritinib, alectinib, brigatinib and lorlatinib) were also appraised. These last four treatments were first appraised for the second line indication, followed by the first line indications (see Fig. 1). Crizotinib also received a positive recommendation for second line treatment.
In 2018, the indication of crizotinib was expanded and included also first- and later line treatment for NSCLC patients with a ROS1 rearrangement. Together with entrectinib, which received a positive recommendation in 2020, these two treatments were the only two drugs recommended for ROS1 positive NSCLC in the UK.
NSCLC with PD-L1 expression
From 2015 onwards, the first technology appraisals for immunotherapies in NSCLC were assessed. Currently, only four compounds (pembrolizumab, nivolumab, atezolizumab and durvalumab) were appraised for a total of eleven indications. These indications can vary with regards to squamous or non-squamous NSCLC, tumour proportion score (TPS) (PD-L1 expression of ≥ 1%; 0–49%; or ≥ 50%), with or without EGFR/ALK mutations, first- or second line, mono- or combination therapy. What stands out is that these pan-tumour drugs are also amenable to targeted therapy for mutations like EGFR and ALK. Overall, second line indications were first appraised, and first line indications followed from 2018 onwards (see Fig. 1).
In the first years of assessing PD-(L)1 inhibitors, more than one appraisal round was needed for an NHS recommendation. It appeared that both companies and NICE needed to find a new way to appraise this immunotherapy category.
Overall trend in treatment landscape
Overall, when a new targeted mutation is identified, it seems that often the first treatment to be appraised by NICE concerns a first line indication when there is a high medical unmet need (see Fig. 1). The expectation for subsequent emerging treatments for the same mutation is that these will first be appraised for the second line indication and that the appraisal of the first line indication will follow consecutively. For new treatments like immunotherapies that focus on a certain expression, there is a high possibility that second line indications will be appraised first as there are already other treatment options available and used when the treatment emerges.
Overview recommendations NICE
Figure 2 provides an overview of NICE recommendations for NSCLC’s technology appraisals; currently, 24 out of 30 appraisals were recommended for NHS (of which four appraisals were previously recommended in CDF), three were recommended for CDF (TA529, TA578, and TA600), and three were not recommended (TA403, TA411, and TA724). All recommendations for NHS and for CDF included market access agreements, varying from commercial agreement (CA) or commercial access agreement (CAA), patient access scheme (PAS), to managed access agreement (MAA). No trend could be found in these commercial arrangements with regards to indications, the treatment’s mechanisms of action, or over time. The content of the commercial arrangements was not public, although for 15 technology appraisals it was mentioned that the CA or PAS concerned a simple price discount.
The above-mentioned three treatments that were not recommended concerned indications for second line ramucirumab (TA403), and both first line necitumumab (TA411) and nivolumab (724). Both treatments ramucirumab and necitumumab showed clinical effectiveness but the most plausible ICERs were well over the range that would normally be considered a cost-effective use of NHS resources. The clinical efficacy for nivolumab in combination with ipilimumab and 2 cycles of chemotherapy has only been compared indirectly with other treatments. These indirect comparisons results were uncertain, and the cost-effectiveness estimates were uncertain and higher than what is considered acceptable. For this treatment it was decided that there is no additional data that could be collected through CDF or from clinical trials to resolve this uncertainty.
Overview of recommendations following NICE technology appraisals of NSCLC treatments for mutations EGFR, ALK and ROS1, for expression PD-L1, and Other. Abbreviations ALK: Anaplastic lymphoma kinase, CA: Commercial Agreement, CDF: Cancer Drug Fund, EGFR: Epidermal growth factor receptor, MAA: Managed Access Agreement, PAS: Patient Access Agreement, PD-L1: Programmed death-ligand 1, ROS1: c-ros oncogene
Cost-effectiveness models
The cost-effectiveness models that were performed until 2016 were mainly (semi-)Markov models or Markov models with ‘area under the curve (AUC) partitioned survival analysis (PartSA) technique’. Increasingly, PartSA models were increasingly used and became a frequently used modelling approach within NSCLC. In Fig. 3, an overview of economic models is presented categorized per treatments and mutation/ expression: PD-L1, EGFR, ALK, ROS1. Out of the 30 technology appraisals, 27 appraisals included three health transitions or states concerning progression free (PF) or pre-progression, progressed disease (PD) or post progression, and death (Fig. 3). A trend that was seen for three technology appraisals of PD-(L)1 inhibitors (TA428, TA584, and TA683) that included these three health transitions, concerned the inclusion of utility values using the proximity to death approach. This implies that more than one death-related time category per health state was included into the model, for example: <30 days or > 30 days to death, or even more time categories like ≤ 5 weeks before death, 5–11 weeks before death, 15–30 weeks before death, > 30 weeks before death. Atezolizumab was appraised for three indications. Notably, the first out of three appraisals used an alternative model by including the following three health states: ‘on treatment’, ‘off treatment’ and ‘death’. Two appraisals included four health transitions (progression free, non-central nervous system (CNS) progression, CNS progression and death) instead of three, these concerned PartSA models for both alectinib and brigatinib for the treatment of first line ALK-positive NSCLC (TA536 and TA670). In this way non-CNS disease and CNS disease could be captured separately.
Overview of economic models used in technology appraisals, categorized as all treatments and per receptor groups: PD-(L)1, EGFR, ALK, ROS1, Other. Abbreviations ALK: Anaplastic lymphoma kinase, AUC: Area under the curve, CNS: Central Nerve System, EGFR: Epidermal growth factor receptor, HS: Health State, OffT: Off Treatment, OnT: On treatment, PD: Progressed Disease, PD-L1: Programmed death-ligand 1, PF: Progression Free, PartSA: Partitioned Survival Analysis, ROS1: c-ros oncogene 1
Cycle lengths for PD-(L)1 inhibitors concerned predominantly seven days (10 out of 11 models), in contrast to models of NSCLC with positive EGFR, ALK or ROS1 mutations. In these models, the majority (13 out of 17 models) applied a cycle length of 28 to 30 days, and the other four technology appraisals applied a cycle length of 7 days. The approach for cycle length seemed to be unrelated to the time horizon. A lifetime horizon was frequently considered with different time horizons often applied as these suffice for a lifetime view in an often-fatal disease (See Table 1; Fig. 4). Over the last decade, the time horizon for economic models for EGFR positive NSCLC increased from 10 years to 20 years for the last technology appraisal concerning first line treatment with osimertinib.
For NSCLC with ALK mutations, the time horizon also started at 10 years and increased to 20 years or higher for the majority of appraisals. Only the two models with four health transitions for first line treatment ALK-positive NSCLC included both a 30-year time horizon. Most of the technology appraisals for PD-(L)1 inhibitors included a time horizon of 20 years, and time horizons of 30 and 40 years were included once, respectively.
There is a tendency for first line treatments to include longer time horizons of 20 to 30 years. In second line treatments, time horizons of 15 years and 20 years were applied most often. Overall, the time horizon included in models increased to 20 years or higher over the years.
The applied WTP threshold in the technology appraisals that focused on first line treatment of EGFR-, ALK- or ROS1-positive NSCLC was £20,000 - £30,000 per QALY overall (Table 1, Appendix B). Only the first treatment available for first line treatment for a specific mutation did meet the NICE end-of-life criteria that led to a WTP threshold of £50,000 per QALY. This concerned crizotinib for first line treatment of ALK positive NSCLC (TA406) and entrectinib for first line treatment of ROS1 positive NSCLC (TA643). All appraisals for targeted second line treatments of EGFR, ALK and ROS1 positive NSCLC met the end-of-life criteria, leading to a WTP threshold of £50,000 per QALY. When looking at the technology appraisals for the PD-(L)1 inhibitors, thresholds showed to be £50,000 per QALY when end-of-life criteria were met, this concerned all first-time appraisals for a certain indication. For PD-(L)1 inhibitors, when another treatment was appraised for the same indication the WTP threshold was £20,000–30,000 per QALY, this not only concerned indications for first but also for second and later lines. Which is not in line with the WTP thresholds for targeted second line treatments like EGFR-, ALK-, and ROS1 inhibitors. In three of these 11 appraisals for PD-(L)1 inhibitors (TA520, TA683, and TA724) the evaluation of end-of-life criteria was performed in two or three subpopulations, leading to separate WTP thresholds per subpopulation.
In short, treatments that were appraised for one targeted population in particular like EGFR-, ALK- or ROS1-positive NSCLC, had one clear WTP threshold. Treatments, like PD-(L)1 inhibitors, that were appraised for an indication that covered multiple subpopulations, the WTP threshold was set per subpopulation depending on meeting the end-of-life criteria.
The results of the ICER were often presented (20 out of 30 appraisals) without stating whether the presented ICER included a confidential discount or not (see Table 1 in Appendix B). Within this group, there were eleven appraisals that presented an ICER that was above the WTP threshold. Also, six of these appraisals received a positive recommendation, two appraisals received a positive recommendation for CDF (TA529 and TA578), and three appraisals received a negative recommendation (TA403, TA411 and TA724 as discussed previously). In the other 10 out of the 30 appraisals, one appraisal presented publicly both the ICER with and without a patient access agreement, one appraisal stated that the list price was included, and the other eight appraisals were only presented with a discount commercial in confidence. Ergo, with the confidentiality involved, it is challenging to describe any further trends with regards to the ICER results.
Although performed for all, data on probabilistic sensitivity analysis as well as scenario analyses were not publicly available for two appraisals (no probabilistic sensitivity analysis available for TA310, and no scenario analyses available for TA258). It is assumed that all appraisals performed uncertainty analyses given data were publicly available on either one or two analyses (univariate, probabilistic or scenario) for the appraisals with unavailable data.
Comparators and indirect comparisons
While the treatment landscape in NSCLC evolved from chemotherapy to targeted therapies and immunotherapy, this was reflected in comparators and subgroups considered in the technology appraisals. Head-to-head trials were often not available for all comparators and subgroups that needed to be included in the technology appraisals. In the 30 appraisals a total of 53 different comparators were included by NICE (Table 2) for 41 assorted indications or subgroups. In only 24 of these cases, comparators were included in head-to-head trials. Indirect comparisons were performed in 22 out of the 30 included appraisals (Table 1 in Appendix C). The methods mixed treatment comparisons (MTC) and indirect analysis were only observed from 2012 to 2016. From 2016 onwards, two new methods were increasingly applied, these concerned network meta-analyses (NMAs) and matching-adjusted indirect comparisons (MAICs). Three indirect treatment comparisons mentioned whether these either unadjusted or adjusted. One ITC and three NMAs concerned fractional polynomials which provided flexible parametric modelling for analysing time-to-event data.
Comments from the ERG (Evidence Review Group) and the NICE committee stated that the MAIC results were uncertain but in some cases were the only way to be able to compare treatments. Often the ERG concluded there is considerable uncertainty with performing indirect comparisons, and the outcomes should be viewed with caution. At the same time these indirect comparisons did support decision making and sometimes the committee concluded that this was the only way to be able to compare.
For twelve out of the 30 appraisals, NICE included two or more comparators. This concerned six appraisals for PD-(L)1 inhibitors (TA428, TA520, TA600, TA683, TA705 and TA724), and appraisals of treatments targeting ALK (TA628 and TA670), EGFR (TA595), ROS1 (TA529), and indications adenocarcinoma (TA347) and NSCLC (TA403). When focusing on inclusion of two or more comparators for PD-(L)1 inhibitors, this was often related to both the TPS (PD-L1 expression of ≥ 1%; 0–49%; or ≥ 50%) and squamous or non-squamous NSCLC status. For example, in TA600 pembrolizumab combined with carboplatin and paclitaxel was appraised for the indication ‘untreated metastatic squamous NSCLC’. To be able to include the preferred comparators, NICE needed to make a distinction in this population between PD-L1 TPS of 0–49%, and 50–100% with no EGFR or ALK mutations. The comparator considered most adequate for the PD-L1 TPS 0–49% population was platinum-based chemotherapy (carboplatin with gemcitabine, or carboplatin with vinorelbine), whereas the correct comparator for the PD-L1 TPS 50–100% concerned pembrolizumab monotherapy. The KEYNOTE-407 clinical trial that was performed to analyse the efficacy for this indication included placebo plus carboplatin and paclitaxel or nab-paclitaxel [46]. The KEYNOTE-042 trial was performed to analyse the efficacy of pembrolizumab monotherapy compared to standard chemotherapy. In this technology appraisal, two ITCs were performed, the first ITC to compare pembrolizumab combination therapy versus four chemotherapy comparators (1. gemcitabine plus cisplatin/carboplatin, 2. paclitaxel/nab-paclitaxel plus cisplatin/carboplatin, 3. docetaxel plus cisplatin/carboplatin, 4. vinorelbine plus cisplatin/carboplatin), whereas the second ITC compared pembrolizumab combination therapy versus pembrolizumab monotherapy. On top of that - separate NMAs were undertaken for: (a) patients with unselected histology and unselected PD-L1 status and (b) squamous histology and unselected PD-L1. Also, separate analyses were presented for progression-free survival (PFS) and overall survival (OS).
In five technology appraisals, the clinical evidence was coming from single arm trials. This concerned three appraisals for second line treatment of ALK positive NSCLC (TA395 in 2016, TA571 in 2019, and TA628 in 2020) and two appraisals for first line treatment of ROS1 positive NSCLC (TA529 in 2018 and TA643 in 2020). Both ITCs and MAICs were performed to be able to compare with the requested comparator in scope by NICE. The exception concerned TA529 of crizotinib for first line treatment of ROS1 positive NSCLC. In this appraisal the clinical trials of crizotinib for the patient population with ALK positive NSCLC were used as a proxy. The committee agreed that using data from a proxy population was not ideal but agreed to explore the proxy data in its decision-making, having considered the relatively small patient population. The committee stated that this should not set a precedent for the use of data from proxy populations in future appraisals.
In the other six appraisals that did not perform indirect comparisons, it was not deemed necessary to perform an indirect comparison as the comparator was included in the clinical trial in scope.
The use of indirect comparisons was common over the last decade. Over time, these indirect comparisons became more established in the form of NMAs and MAICs compared to the simpler comparisons that were conducted in the earlier years of the last decade.
Patient reported outcome measures
All technology appraisals needed to include patient reported outcomes in the form of EuroQol-5 Dimension-3L (EQ-5D-3L) in the cost-effectiveness model. It appeared that use of EQ-5D in the economic models was predominantly in line with the NICE guidelines. Often other patient reported outcome measures (PROMs) were also considered in the technology appraisal to be able to further assess on lung cancer disease in specific, although these outcomes were mostly not included in the cost-effectiveness model. After EQ-5D, the PROMs EORTC core quality of life questionnaires C-30 (EORTC QLQ-C30) and LC-13 (EORTC QLQ-LC13) were most frequently included in clinical trials since 2015. Other PROMs that were used several times concerned the Lung Cancer symptom scale (LCSS) and the Functional Assessment of Cancer Therapy-Lung (FACT-L).
Discussion
Over the last decade, 30 technology appraisals were performed for innovative NSCLC treatments in the UK. All these appraisals concerned NSCLC in the advanced and/or metastatic setting. Overall, 80% of appraisals were recommended for NHS, in addition three appraisals were recommended for use in CDF, and three appraisals were not recommended. Observed trends included first time appraisals for new mutations treated with targeted therapies concerned first line indications and for immunotherapy concerned second line; PartSA models were most frequently used and included most commonly three health transitions concerning PF, PD and death over a lifetime horizon with a cycle length of 7 days for immunotherapy and 28 to 30 days for targeted treatments; a total of 53 different comparators were included by NICE and 22 out of the 30 appraisals included indirect comparisons as not all comparators were investigated in head-to-head trials and for five appraisals the clinical evidence was coming from single arm trials; all appraisals included EQ-5D outcomes in the cost-effectiveness model and other patient reported outcomes that were considered most frequently concerned EORTC QLQ-C30 and LC-13.
Even though all new cancer drugs are considered for recommendation into the CDF, in practice not all new treatments were recommended for CDF uptake as 80% of appraisals were directly recommended for NHS. Recommendation for CDF was only the case when there was significant remaining clinical uncertainty which indirectly could lead to uncertainty around the end-of-life criteria and the cost-effectiveness.
Over these ten years, various methods to evaluate the cost-effectiveness of treatments were reported. With regards to cost-effectiveness models, the NICE Decision Support Unit (DSU) published a Technical Support Document (TSD 14) in 2011 in which it stated the preference of using PartSA modelling for cancer treatments [47]. It took until 2019 before most of the appraised cost-effectiveness models were in line with this recommendation in the field of NSCLC. However, PartSA models with the health transitions progression free, progressed disease and death became the cornerstone of health economic modelling in NSCLC. For the appraisals of PD-(L)1-inhibitors a possible new trend was seen with the implementation of the proximity of death approach. As not all technology appraisals for PD-(L)1 inhibitors used these time categories, it is not certain whether this will become a new trend within the appraisals of immunotherapies for NSCLC. Increasingly, treatment models for ALK positive NSCLC were more specifically designed for this individual mutation with applying a four health states model instead of three health states. Which is in line with the clinical trial endpoints of non-CNS progression and CNS progression. This could be an important new trend for the upcoming models for the treatment of NSCLC with ALK mutations, as CNS progression is a major cause of illness and death in this mutation specifically [48, 49]. Even though the perspective was mostly life long, we notified longer time horizons with up to 30 years for first line therapy and up to 20 years for second line, which is in line with the trend of improved survival in clinical trials. Cycle lengths differed from seven days for PD-(L)1 inhibitors, in line with administration every three or six weeks intravenously, to 28 or 30 days for inhibitors of the mutations EGFR, ALK or ROS1, which are administered orally daily. Our assumption was confirmed that when treatments for new mutations become available, NICE will first consider a WTP threshold of £50,000 per QALY. Our review showed that when more treatments for the same mutation will come to market, the WTP threshold for first line treatment decreased to £20,000–30,000 per QALY and for second line treatment was £50,000 per QALY as these were meeting the end-of-life criteria.
It was difficult to analyse ICER results as these were often only partially public and it was frequently unclear whether the presented ICER outcomes included a confidential discount. Therefore, the ICER results would be informative even though it would often not be the definite ICER for the intervention at scope.
Indirect comparisons were often conducted in the technology appraisals for NSCLC as direct head-to-head trials were often lacking for the various comparators or the subgroups that needed to be included for the appraisal. Although we expected that over time indirect comparisons would become more frequently applied due to the lack of active comparators, these comparisons were already performed in the earlier years of the last decade. Over time ITCs such as NMAs and MAICs became more established in comparison to the mixed treatment comparison and to more simple indirect comparisons. Especially since the first technology appraisals for immunotherapies in 2015 the treatment comparisons were getting more and more established. This complexity was due to the different indications and the various patient populations included in a technology appraisal, and the fast pace of updates in standard of care within NSCLC. This is in line with other disease areas within oncology where in a short period of time new treatments emerged that became the standard of care, for example in HER2-positive metastatic breast cancer and melanoma. [50,51,52,53]
Use of EQ5D in the economic models was predominantly in line with the NICE guidelines, and PROMs more focused on the quality of life in oncology and lung oncology were often captured by QLQ-C30 and QLQ-LC13 from 2015 onwards.
Strengths and limitations
One of the strengths of this review is that we captured the finalised technology appraisals in the NICE database with a timespan of one decade (January 1st, 2012 - January 1st, 2022), enabling us to investigate the up-to-date trends of NSCLC treatments in the UK. However, we also have some limitations in this review. Firstly, our analysis only focused on the technology appraisals of NICE and therefore appraisals in journals and in other countries were not considered. Secondly, a challenge of this analysis was that the NICE database contains multiple documents and sometimes multiple review rounds were included, or two rounds of technology appraisals were necessary to reach the final appraisal determination. In these documents various perspectives and elaborations on certain topics were included from the Committee, the ERG, and the company. This made it very challenging to capture the proper information for our data extraction and in our analysis. Data from each technology appraisal was extracted from the most recent set of relevant papers from NICE. When specific data was not found in the most recent papers due to redacted text and data (black boxes) or references to earlier appraisal rounds, additional data was extracted from former available committee papers. Sometimes information could not be found in all the appraisal rounds due to confidentiality and redacted text. This limits our analyses of specific data or trends, but - we feel - not invalidates our general findings.
Recommendations for future studies, policy, and practice
The technology appraisals that were needed to make decisions were both challenging for companies, ERG and NICE. This field is getting even more complex as new mutations and expressions are still being identified and innovative therapies are emerging within the field of NSCLC, such as treatments for the mutations or expressions BRAF, HER2, HER3, KRAS, MET, NTRK, RET, and TROP2. Some treatments are also pan-tumour molecular targets. Also, the appraisals over the last ten years included NSCLC in the advanced and/or metastatic setting. Currently, treatments are showing efficacy in the adjuvant and neo-adjuvant treatment setting, which will result in new treatment possibilities and therefore appraisals beyond the scope of advanced and/or metastatic NSCLC. It will be interesting to analyse future appraisals of advanced and/or metastatic NSCLC treatments considering these treatment options in earlier stages of NSCLC, together with the effect of lung cancer screening roll out leading to earlier diagnosis and improved outcomes in the UK [54].
Hence, in the years to come, a lot of new technology appraisals need to be performed not only just for the NSCLC disease area but also for overarching disease areas. It is not feasible to have one or two clinical trials for just one targeted treatment in NSCLC that will capture all the different patient (sub)populations or the different comparator treatments needed per subgroup, or to capture the current standard of care in such a rapidly changing disease area. Also, what is an acceptable way of appraising drugs for these oncogenes that will first have to be evaluated in single arm trials as there is currently no comparator? This leads to obstacles in the appraisal process with regards to clinical and scientific robustness and the timely matter in which these appraisals need to be performed. Therefore, the importance of indirect comparisons will increase even more. Our recommendation is to create more guidance for the elaboration of indirect comparisons on top of the existing guidance, like NICE DSU TSD 2 and 18, in order to reach more standardisation and transparency between appraisals, and that it is known which approaches and methods are acceptable [55, 56].
It can be questioned whether NICE needs a new approach with regards to appraising the emerging pan-tumour indications. NICE already recommended larotrectinib for use on the CDF for a range of cancers with the NTRK gene fusion mutation in May 2020 [57]. Other pan-tumour treatments are still being appraised per indication. One of the challenges that will also arise, is the need for including a recommended pan-tumour treatment as comparator in an appraisal for a targeted therapy in NSCLC. Further research needs to be performed to identify the obstacles and opportunities around this.
We have found that the patient reported outcomes used in the models were in line with the requirements of NICE. A deeper analysis into the use of PROMs is needed to further identify the trends and whether the impact of treatments on the quality of life of NSCLC patients can be captured in general with the required EQ5D utility values.
As already mentioned in this analysis, technology appraisals became more complicated. Further research is important to identify in what way the technology appraisal process can be simplified and be made more transparent and reproducible. NICE announced in January 2022 that it has rolled out changes to simplify and streamline processes to allow for more flexible decision making. Given the findings of this analysis this seems a great step in the right direction in this dynamic and challenging playing field [58].
Conclusion
This study showed that over the last decade technology appraisals in the field of NSCLC became more complex due to the emergence of targeted therapies and immunotherapies that led to multiple different indications, subpopulations and comparators that needed to be included in appraisals, and the fast pace of updates in standard of care within NSCLC. Within the appraisals, PartSA models with three health states (PF, PD, Death) became the cornerstone within NSCLC, with time horizons up to 30 years and frequent use of NMAs and MAICs for indirect comparisons. Finally, with 90% positive recommendations for uptake either in NHS or CDF, the majority of treatments was recommended.
Data availability
All data used in this analysis were retrieved from public sources. Our data collection is available upon request.
References
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., et al.: Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021)
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E.F., Faivre-Finn, C., et al.: Metastatic non-small cell lung cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv192–237 (2018)
Chen, R., Manochakian, R., James, L., Azzouqa, A.G., Shi, H., Zhang, Y., et al.: Emerging therapeutic agents for advanced non-small cell lung cancer. J. Hematol. Oncol. 13(1), 58 (2020)
Prescott, J., Dilkes, J., George, E., Sutcliffe, F., Boswell, C., Kenyon, A., et al.: Guide to the processes of technology appraisal [Internet]. 2014 [cited 2022 May 28]. https://www.nice.org.uk/process/pmg19/resources/guide-to-the-processes-of-technology-appraisal-pdf-72286663351237
NHS England Cancer Drugs Fund Team: Appraisal and Funding of Cancer Drugs from July 2016 (including the new Cancer Drugs Fund) [Internet]. 2016 Jul [cited 2022 Mar 28]. https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making
Boysen, M., Robertson, J., Woodward, L., Longson, C., Stevens, A., Adler, A.: nice.org.uk. 2013. Guide to the methods of technology appraisal 2013
Gallacher, D., Auguste, P., Connock, M.: How do Pharmaceutical companies Model Survival of Cancer patients? A review of NICE single technology appraisals in 2017. Int. J. Technol. Assess. Health Care. 35(2), 160–167 (2019)
Bullement, A., Meng, Y., Cooper, M., Lee, D., Harding, T.L., O’Regan, C., et al.: A review and validation of overall survival extrapolation in health technology assessments of cancer immunotherapy by the National Institute for Health and Care Excellence: How did the initial best estimate compare to trial data subsequently made available? J. Med. Econ. 22(3), 205–214 (2019)
Bell Gorrod, H., Kearns, B., Stevens, J., Thokala, P., Labeit, A., Latimer, N., et al.: A review of survival analysis methods used in NICE technology appraisals of Cancer treatments: Consistency, limitations, and Areas for Improvement. Med. Decis. Making. 39(8), 899–909 (2019)
Murphy, P., Glynn, D., Dias, S., Hodgson, R., Claxton, L., Beresford, L., et al.: Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: A systematic review and decision-framework. Health Technol. Assess. (Rockv). 25(76), 1–228 (2021)
Gallacher, D., Auguste, P., Royle, P., Mistry, H., Armoiry, X.: A systematic review of economic evaluations assessing the cost-effectiveness of licensed drugs used for previously treated epidermal growth factor receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) negative Advanced/Metastatic Non-small Cell Lung Cancer. Clin. Drug Investig. 39(12), 1153–1174 (2019)
Nguyen, C.T.T., Petrelli, F., Scuri, S., Nguyen, B.T., Grappasonni, I.: A systematic review of pharmacoeconomic evaluations of erlotinib in the first-line treatment of advanced non-small cell lung cancer. Eur. J. Health Econ. 20(5), 763–777 (2019)
Verma, V., Sprave, T., Haque, W., Simone, C.B., Chang, J.Y., Welsh, J.W., et al.: A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother Cancer. 6(1), 128 (2018)
Qiao, N., Insinga, R., de Lima Lopes Junior, G., Cook, J., Sénécal, M.: A review of cost-effectiveness studies of Pembrolizumab regimens for the treatment of Advanced Non-small Cell Lung Cancer. Pharmacoecon Open. 5(3), 365–383 (2021)
Jäkel, A., Plested, M., Dharamshi, K., Modha, R., Bridge, S., Johns, A.: A Systematic Review of Economic Evaluations in second and later lines of therapy for the treatment of Non-small Cell Lung Cancer. Appl. Health Econ. Health Policy. 11(1), 27–43 (2013)
NICE: nice.org.uk. [cited 2023 May 29]. Erlotinib for the first-line treatment of locally advanced or metastatic EGFR-TK mutation-positive non-small-cell lung cancer; Technology appraisal guidance [TA258] [Internet] (2012). https://www.nice.org.uk/guidance/ta258/history
NICE: nice.org.uk. [cited 2022 Jan 27]. Afatinib for treating epidermal growth factor receptor mutation-positive locally advanced or metastatic non-small-cell lung cancer; Technology appraisal guidance [TA310] [Internet] (2014). https://www.nice.org.uk/guidance/ta310
NICE: nice.org.uk. [cited 2022 Jan 27]. Nintedanib for previously treated locally advanced, metastatic, or locally recurrent non-small-cell lung cancer; Technology appraisal guidance [TA347] [Internet] (2015). https://www.nice.org.uk/guidance/ta3470
NICE: nice.org.uk. [cited 2022 Jan 27]. Ceritinib for previously treated anaplastic lymphoma kinase positive non-small-cell lung cancer; Technology appraisal guidance [TA395] [Internet] (2016). https://www.nice.org.uk/guidance/ta395
NICE: nice.org.uk. [cited 2022 Jan 27]. Pemetrexed maintenance treatment for non-squamous non-small-cell lung cancer after pemetrexed and cisplatin; Technology appraisal guidance [TA402] [Internet] (2016). https://www.nice.org.uk/guidance/ta402
NICE: nice.org.uk. [cited 2022 Jan 27]. Ramucirumab for previously treated locally advanced or metastatic non-small-cell lung cancer; Technology appraisal guidance [TA403] [Internet] (2016). https://www.nice.org.uk/guidance/ta403
NICE: nice.org.uk. [cited 2022 Jan 27]. Crizotinib for untreated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA406] [Internet] (2016). https://www.nice.org.uk/guidance/ta406
NICE: nice.org.uk. [cited 2022 Jan 27]. Necitumumab for untreated advanced or metastatic squamous non-small-cell lung cancer; Technology appraisal guidance [TA411] [Internet] (2016). https://www.nice.org.uk/guidance/ta411
NICE: nice.org.uk. [cited 2022 Jan 27]. Crizotinib for previously treated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA422] [Internet] (2016). https://www.nice.org.uk/guidance/ta422
NICE: nice.org.uk. [cited 2022 Jan 27]. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy; Technology appraisal guidance [TA428] [Internet] (2017). https://www.nice.org.uk/guidance/ta428/history
NICE: nice.org.uk. [cited 2022 Jan 27]. Ceritinib for untreated ALK-positive non-small-cell lung cancer; Technology appraisal guidance [TA500] [Internet] (2018). https://www.nice.org.uk/guidance/ta500
NICE: nice.org.uk. [cited 2022 Jan 27]. Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy; Technology appraisal guidance [TA520] [Internet] (2018). https://www.nice.org.uk/guidance/ta520
NICE: nice.org.uk. [cited 2022 Jan 27]. Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer; Technology appraisal guidance [TA531] [Internet] (2018). https://www.nice.org.uk/guidance/ta531
NICE: nice.org.uk. [cited 2022 Jan 27]. Crizotinib for treating ROS1-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA529] [Internet] (2018). https://www.nice.org.uk/guidance/ta529
NICE: nice.org.uk. [cited 2022 Jan 27]. Alectinib for untreated ALK-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA536] [Internet] (2018). https://www.nice.org.uk/guidance/ta536
NICE: nice.org.uk. [cited 2022 Jan 27]. Brigatinib for treating ALK-positive advanced non-small-cell lung cancer after crizotinib; Technology appraisal guidance [TA571] [Internet] (2019). https://www.nice.org.uk/guidance/ta571
NICE: nice.org.uk. [cited 2022 Jan 27]. Durvalumab for treating locally advanced unresectable non-small-cell lung cancer after platinum-based chemoradiation; Technology appraisal guidance [TA578] [Internet] (2019). https://www.nice.org.uk/guidance/ta578
NICE: nice.org.uk. [cited 2022 Jan 27]. Atezolizumab in combination for treating metastatic non-squamous non-small-cell lung cancer; Technology appraisal guidance [TA584] [Internet] (2019). https://www.nice.org.uk/guidance/ta584
NICE: nice.org.uk. [cited 2022 Jan 27]. Dacomitinib for untreated EGFR mutation-positive non-small-cell lung cancer; Technology appraisal guidance [TA595] [Internet] (2019). https://www.nice.org.uk/guidance/ta595
NICE: nice.org.uk. [cited 2022 Jan 27]. Pembrolizumab with carboplatin and paclitaxel for untreated metastatic squamous non-small-cell lung cancer; Technology appraisal guidance [TA600] [Internet] (2019). https://www.nice.org.uk/guidance/ta600
NICE: nice.org.uk. [cited 2022 Jan 27]. Lorlatinib for previously treated ALK-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA628] [Internet] (2020). https://www.nice.org.uk/guidance/ta628
NICE: nice.org.uk. [cited 2022 Jan 27]. Entrectinib for treating ROS1-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA643] [Internet] (2020). https://www.nice.org.uk/guidance/ta643
NICE: nice.org.uk. [cited 2022 Jan 27]. Osimertinib for treating EGFR T790M mutation-positive advanced non-small-cell lung cancer; Technology appraisal guidance [TA653] [Internet] (2020). https://www.nice.org.uk/guidance/ta653
NICE: nice.org.uk. Osimertinib for untreated EGFR mutation-positive non-small-cell lung cancer; Technology appraisal guidance [TA654] (2020)
NICE: nice.org.uk. [cited 2022 Jan 27]. Nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy; Technology appraisal guidance [TA655] [Internet] (2020). https://www.nice.org.uk/guidance/ta655
NICE: nice.org.uk. [cited 2022 Jan 27]. Brigatinib for ALK-positive advanced non-small-cell lung cancer that has not been previously treated with an ALK inhibitor; Technology appraisal guidance [TA670] [Internet] (2021). https://www.nice.org.uk/guidance/ta670
NICE: nice.org.uk. [cited 2022 Jan 27]. Pembrolizumab with pemetrexed and platinum chemotherapy for untreated, metastatic, non-squamous non-small-cell lung cancer; Technology appraisal guidance [TA683] [Internet] (2021). https://www.nice.org.uk/guidance/ta683
NICE: nice.org.uk. [cited 2022 Jan 27]. Atezolizumab monotherapy for untreated advanced non-small-cell lung cancer; Technology appraisal guidance [TA705] [Internet] (2021). https://www.nice.org.uk/guidance/ta705
NICE: nice.org.uk. [cited 2022 Jan 27]. Nivolumab for advanced non-squamous non-small-cell lung cancer after chemotherapy; Technology appraisal guidance [TA713] [Internet] (2021). https://www.nice.org.uk/guidance/ta713
NICE: nice.org.uk. [cited 2022 Jan 27]. Nivolumab with ipilimumab and chemotherapy for untreated metastatic non-small-cell lung cancer; Technology appraisal guidance [TA724] [Internet] (2021). https://www.nice.org.uk/guidance/ta724
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gümüş, M., Mazières, J., et al.: Pembrolizumab plus Chemotherapy for squamous non–small-cell Lung Cancer. N. Engl. J. Med. 379(21), 2040–2051 (2018)
Decision Support Unit, Latimer, N., NICE DSU TECHNICAL SUPPORT, DOCUMENT 14: SURVIVAL ANALYSIS FOR ECONOMIC EVALUATIONS ALONGSIDE CLINICAL TRIALS - EXTRAPOLATION WITH PATIENT-LEVEL DATA [Internet], Sheffield, U.K.: Jun [cited 2022 Aug 7]. (2011). https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf
Ali, A., Goffin, J.R., Arnold, A., Ellis, P.M.: Survival of patients with non-small-cell Lung Cancer after a diagnosis of Brain metastases. Curr. Oncol. 20(4), 300–306 (2013)
Venur, V.A., Ahluwalia, M.S.: Targeted Therapy in Brain Metastases: Ready for Primetime? American Society of Clinical Oncology Educational Book. ;(36):e123–30. (2016)
NICE. nice.org.uk: [cited 2022 Sep 29]. Tucatinib with trastuzumab and capecitabine for treating HER2-positive advanced breast cancer after 2 or more anti-HER2 therapies. (2022). https://www.nice.org.uk/guidance/ta786/history
NICE. nice.org.uk: [cited 2023 May 28]. Trastuzumab deruxtecan for treating HER2-positive unresectable or metastatic breast cancer after 2 or more anti-HER2 therapies. (2021). https://www.nice.org.uk/guidance/ta704/history
NICE. nice.org.uk: [cited 2023 May 28]. Pembrolizumab for advanced melanoma not previously treated with ipilimumab. (2015). https://www.nice.org.uk/guidance/ta366
NICE. nice.org.uk: [cited 2022 Sep 29]. Nivolumab for treating advanced (unresectable or metastatic) melanoma. (2016). https://www.nice.org.uk/guidance/ta384/history
Cancer Research, U.K.: (2024). https://www.cancerresearchuk.org/health-professional/cancer-screening/lung-cancer-screening. Announcement Lung Cancer Screening
Unit, D.S., Dias, S., Welton, N.J., Sutton, A., Ades, A., Nice Dsu Technical Support, Document 2: A Generalised Linear Modelling Framework For Pairwise And Network Meta-Analysis Of Randomised Controlled Trials [Internet]:. Sheffield, UK; Aug [cited 2022 Aug 7]. (2011). https://www.ncbi.nlm.nih.gov/books/NBK310366/pdf/Bookshelf_NBK310366.pdf
Phillippo, D., Ades, T., Dias, S., Palmer, S., Abrams, K.R., Welton, N.: NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. [Internet]. Sheffield, UK; 2016 Dec [cited 2022 Aug 7]. https://research-information.bris.ac.uk/ws/portalfiles/portal/94868463/Population_adjustment_TSD_FINAL.pdf
NICE: nic.org.uk. 2020 [cited 2022 Jun 24]. Larotrectinib for treating NTRK fusion-positive solid tumours. https://www.nice.org.uk/guidance/ta630
NICE: nice.org.uk. [cited 2022 Sep 24]. Changes we’re making to health technology evaluation. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/changes-to-health-technology-evaluation
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception, design and analysis of the work and the data. Research & analysis phase was performed and supported by LW, SW, GZ, and AP. LW drafted and GZ, SW, AP, JB, CB, MJP and JN revised the work. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
LW is currently employed at AstraZeneca, this research has been performed unrelated to her work at AstraZeneca and is part of her PhD research at the University Medical Center Groningen, the Netherlands. LW is a previous employee (at time of research) of Asc Academics Inc. SW and AP are employees of Asc Academics BV. GZ has no conflict of interest. MP received grants and honoraria from various pharmaceutical companies, including companies interested in the subject of this paper. JB received consultancy fees, honorarium, and research funding from Aardex, AstraZeneca, Chiesi, GSK, Novartis, Pfizer, Pill Connect, Teva, Trudell Medical and Vertex to consult, give lectures, provide advice, and conduct independent research, all paid to his institution. CB received grants and honoraria from various pharmaceutical companies, including companies interested in the subject of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Westerink, L., Wolters, S., Zhou, G. et al. Trends in NICE technology appraisals of non-small cell lung cancer drugs over the last decade. Eur J Health Econ (2024). https://doi.org/10.1007/s10198-024-01711-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10198-024-01711-0