Abstract
Background
Non-small cell lung cancer (NSCLC) is one of the most commonly diagnosed cancers. There are many published studies of cost-effectiveness analyses of licensed treatments, but no study has compared these studies or their approaches simultaneously.
Objective
To investigate the methodology used in published economic analyses of licensed interventions for previously treated advanced/metastatic NSCLC in patients without anaplastic lymphoma kinase or epidermal growth factor receptor expression.
Methods
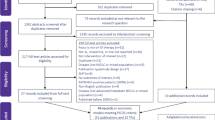
A systematic review was performed, including a systematic search of key databases (e.g. MEDLINE, EMBASE, Web of Knowledge, Cost-effectiveness Registry) limited to the period from 01 January 2001 to 26 July 2019. Two reviewers independently screened, extracted data and quality appraised identified studies. The reporting quality of the studies was assessed by using the Consolidated Health Economic Evaluation Reporting Standards and the Philips’ checklists.
Results
Thirty-one published records met the inclusion criteria, which corresponded to 30 individual cost-effectiveness analyses. Analytical approaches included partitioned survival models (n = 14), state-transition models (n = 7) and retrospective analyses of new or published data (n = 8). Model structure was generally consistent, with pre-progression, post-progression and death health states used most commonly. Other characteristics varied more widely, including the perspective of analysis, discounting, time horizon, usually to align with the country that the analysis was set in.
Conclusions
There are a wide range of approaches in the modelling of treatments for advanced NSCLC; however, the model structures are consistent. There is variation in the exploration of sensitivity analyses, with considerable uncertainty remaining in most evaluations. Improved reporting is necessary to ensure transparency in future analyses.

Similar content being viewed by others
References
Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. 12 September 2018; Available from: https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf. Cited 30 Aug 2019.
Lung cancer clinical outcomes publication 2017 (for surgical operations performed in 2015). Royal College of Physicians. 2017.
Armoiry X, Tsertsvadze A, Connock M, Royle P, Melendez-Torres GJ, Souquet P, et al. Comparative efficacy and safety of licensed treatments for previously treated non-small cell lung cancer: a systematic review and network meta-analysis. PLoS One. 2018;13(7):e0199575.
Connock M, Armoiry X, Tsertsvadze A, Melendez-Torres GJ, Royle P, Andronis L, et al. Comparative survival benefit of currently licensed second or third line treatments for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) negative advanced or metastatic non-small cell lung cancer: a systematic review and secondary analysis of trials. BMC Cancer. 2019;19(1):392.
Schmidt C. The benefits of immunotherapy combinations. Nature. 2017;552(7685):S67–9.
Créquit P, Chaimani A, Yavchitz A, Attiche N, Cadranel J, Trinquart L, et al. Comparative efficacy and safety of second-line treatments for advanced non-small cell lung cancer with wild-type or unknown status for epidermal growth factor receptor: a systematic review and network meta-analysis. BMC Med. 2017;15(1):193.
Armoiry XMH, Royle P, Auguste P, Gallacher D. A systematic review of the use of economic evaluations to assess the cost-effectiveness of licensed drugs used in advanced/metastatic non-small cell lung cancer. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=88805. Cited 30 Aug 2019.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 2009-07-21 10:46:49–339.
Belani CP, Eckardt J. Development of docetaxel in advanced non-small-cell lung cancer. Lung Cancer (Amsterdam Netherlands). 2004;46(Suppl 2):S3–11.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049.
Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess (Winchester, England). 2004;8(36):iii–iv, ix–xi, 1–158.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
Leighl NB, Shepherd FA, Kwong R, Burkes RL, Feld R, Goodwin PJ. Economic analysis of the TAX 317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small-cell lung cancer. J Clin Oncol. 2002;20(5):1344–52.
Holmes J, Dunlop D, Hemmett L, Sharplin P, Bose U. A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small cell lung cancer. PharmacoEconomics. 2004;22(9):581–9.
NICE. Pemetrexed for the treatment of non-small-cell lung cancer, Technology appraisal guidance [TA124]. 2007; Available from: https://www.nice.org.uk/guidance/ta124. Cited 27 Feb 2019.
Araujo A, Parente B, Sotto-Mayor R, Teixeira E, Almodovar T, Barata F, et al. An economic analysis of erlotinib, docetaxel, pemetrexed and best supportive care as second or third line treatment of non-small cell lung cancer. Revista portuguesa de pneumologia. 2008;14(6):803–27.
Carlson JJ, Reyes C, Oestreicher N, Lubeck D, Ramsey SD, Veenstra DL. Comparative clinical and economic outcomes of treatments for refractory non-small cell lung cancer (NSCLC). Lung Cancer (Amsterdam, Netherlands). 2008;61(3):405–15.
McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al. Erlotinib for the treatment of relapsed non-small cell lung cancer. Health Technol Assess (Winchester, Engl). 2009;13(Suppl 1):41–7.
Lewis G, Peake M, Aultman R, Gyldmark M, Morlotti L, Creeden J, et al. Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small-cell lung cancer in the United Kingdom. J Int Med Res. 2010;38(1):9–21.
Asukai Y, Valladares A, Camps C, Wood E, Taipale K, Arellano J, et al. Cost-effectiveness analysis of pemetrexed versus docetaxel in the second-line treatment of non-small cell lung cancer in Spain: results for the non-squamous histology population. BMC Cancer. 2010;29(10):26.
Cromwell I, van der Hoek K, Melosky B, Peacock S. Erlotinib or docetaxel for second-line treatment of non-small cell lung cancer: a real-world cost-effectiveness analysis. J Thorac Oncol. 2011;6(12):2097–103.
Vergnenegre A, Corre R, Berard H, Paillotin D, Dujon C, Robinet G, et al. Cost-effectiveness of second-line chemotherapy for non-small cell lung cancer: an economic, randomized, prospective, multicenter phase III trial comparing docetaxel and pemetrexed: the GFPC 05-06 study. J Thorac Oncol. 2011;6(1):161–8.
Cromwell I, van der Hoek K, Malfair Taylor SC, Melosky B, Peacock S. Erlotinib or best supportive care for third-line treatment of advanced non-small-cell lung cancer: a real-world cost-effectiveness analysis. Lung Cancer (Amsterdam, Netherlands). 2012;76(3):472–7.
Greenhalgh J, Bagust A, Boland A, Dwan K, Beale S, Hockenhull J, et al. Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE technology appraisals 162 and 175): a systematic review and economic evaluation. Health Technol Assess (Winchester, Engl). 2015;19(47):1–134.
NICE. Nintedanib for previously treated locally advanced, metastatic, or locally recurrent non-small-cell lung cancer, Technology appraisal guidance [TA347]. 2015; Available from: https://www.nice.org.uk/guidance/ta347. Cited 27 Feb 2019.
Espinosa Bosch M, Asensi Diez R, Garcia Agudo S, Clopes EA. Nintedanib in combination with docetaxel for second-line treatment of advanced non-small-cell lung cancer; GENESIS-SEFH drug evaluation report. Farmacia hospitalaria: organo oficial de expresion cientifica de la Sociedad Espanola de Farmacia Hospitalaria. 2016;40(4):316–27.
Matter-Walstra K, Schwenkglenks M, Aebi S, Dedes K, Diebold J, Pietrini M, et al. A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol. 2016;11(11):1846–55.
Goeree R, Villeneuve J, Goeree J, Penrod JR, Orsini L, Tahami Monfared AA. Economic evaluation of nivolumab for the treatment of second-line advanced squamous NSCLC in Canada: a comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med Econ. 2016;19(6):630–44.
NICE. Ramucirumab for previously treated locally advanced or metastatic non-small-cell lung cancer, Technology appraisal guidance [TA403]. 2016; Available from: https://www.nice.org.uk/guidance/ta403. Cited 27 Feb 2019.
Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, et al. Cost-effectiveness of pembrolizumab versus docetaxel for the treatment of previously treated PD-L1 positive advanced NSCLC patients in the United States. J Med Econ. 2017;20(2):140–50.
Pignata M, Chouaid C, Le Lay K, Luciani L, McConnachie C, Gordon J, et al. Evaluating the cost-effectiveness of afatinib after platinum-based therapy for the treatment of squamous non-small-cell lung cancer in France. Clin Econ Outcome Res. 2017;9:655–68.
NICE. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy, Technology appraisal guidance [TA428]. 2017; Available from: https://www.nice.org.uk/guidance/ta428. Cited 27 Feb 2019.
NICE. Nivolumab for previously treated squamous non-small-cell lung cancer, Technology appraisal guidance [TA483]. 2017; Available from: https://www.nice.org.uk/guidance/ta483. Cited 27 Feb 2019.
NICE. Nivolumab for previously treated non-squamous non-small-cell lung cancer, Technology appraisal guidance [TA484]. 2017; Available from: https://www.nice.org.uk/guidance/ta484. Cited 27 Feb 2019.
NICE. Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy, Technology appraisal guidance [TA520]. 2018; Available from: https://www.nice.org.uk/guidance/ta520. Cited 27 Feb 2019.
Aguiar P Jr, Giglio AD, Perry LA, Penny-Dimri J, Babiker H, Tadokoro H, et al. Cost-effectiveness and budget impact of lung cancer immunotherapy in South America: strategies to improve access. Immunotherapy. 2018;10(10):887–97.
Guirgis HM. The impact of PD-L1 on survival and value of the immune check point inhibitors in non-small-cell lung cancer; proposal, policies and perspective. J Immunotherap Cancer. 2018;6(1):15.
Shafrin J, Skornicki M, Brauer M, Villeneuve J, Lees M, Hertel N, et al. An exploratory case study of the impact of expanding cost-effectiveness analysis for second-line nivolumab for patients with squamous non-small cell lung cancer in Canada: does it make a difference? Health Policy (Amsterdam, Netherlands). 2018;122(6):607–13.
Zhu J, He W, Ye M, Fu J, Chu YB, Zhao YY, et al. Cost-effectiveness of afatinib and erlotinib as second-line treatments for advanced squamous cell carcinoma of the lung. Fut Oncol (Lond, Engl). 2018;14(27):2833–40.
Gao L, Li SC. Modelled economic evaluation of nivolumab for the treatment of second-line advanced or metastatic squamous non-small-cell lung cancer in Australia using both partition survival and Markov models. Appl Health Econ Health Policy. 2019;17(3):371–80.
Merino Almazan M, Duarte Perez JM, Marin Pozo JF, Ortega Granados AL, Muros De Fuentes B, Quesada Sanz P, et al. A multicentre observational study of the effectiveness, safety and economic impact of nivolumab on non-small-cell lung cancer in real clinical practice. Int J Clin Pharm. 2019;41(1):272–9.
Ondhia U, Conter HJ, Owen S, Zhou A, Nam J, Singh S, et al. Cost-effectiveness of second-line atezolizumab in Canada for advanced non-small cell lung cancer (NSCLC). J Med Econ. 2019;22(7):625–37.
Woods B, Sideris E, Palmer S, Latimer N, Soares M. NICE DSU Technical Support Document 19. Partitioned survival analysis for decision modelling in health care: a critical review. 2017; Available from: http://www.nicedsu.org.uk. Cited 27 Feb 2019.
Garattini S, Bertele’ V. Ethics in clinical research. J Hepatol. 2009;51(4):792–7.
Gallacher D, Auguste P, Connock M. How do pharmaceutical companies model survival of cancer patients? A review of NICE single technology appraisals in 2017. Int J Technol Assess Health Care. 2019;35(2):160–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the University of Warwick Research Development Fund (Warwick Medical School, December 2017).
Conflict of Interest
Daniel Gallacher, Peter Auguste, Pamela Royle, Hema Mistry and Xavier Armoiry have no conflict of interest to decalre.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gallacher, D., Auguste, P., Royle, P. et al. A Systematic Review of Economic Evaluations Assessing the Cost-Effectiveness of Licensed Drugs Used for Previously Treated Epidermal Growth Factor Receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) Negative Advanced/Metastatic Non-Small Cell Lung Cancer. Clin Drug Investig 39, 1153–1174 (2019). https://doi.org/10.1007/s40261-019-00859-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00859-5