Abstract
Objectives
Comparative economic assessments of renal replacement therapies (RRT) are common and often used to inform national policy in the management of end-stage renal disease (ESRD). This study aimed to assess existing cost-effectiveness analyses of dialysis modalities and consider whether the methods applied and results obtained reflect the complexities of the real-world treatment pathway experienced by ESRD patients.
Methods
A systematic literature review (SLR) was conducted to identify cost-effectiveness studies of dialysis modalities from 2005 onward by searching Embase, MEDLINE, EBM reviews, and EconLit. Economic evaluations were included if they compared distinct dialysis modalities (e.g. in-centre haemodialysis [ICHD], home haemodialysis [HHD] and peritoneal dialysis [PD]).
Results
In total, 19 cost-effectiveness studies were identified. There was considerable heterogeneity in perspectives, time horizon, discounting, utility values, sources of clinical and economic data, and extent of clinical and economic elements included. The vast majority of studies included an incident dialysis patient population. All studies concluded that home dialysis treatment options were cost-effective interventions.
Conclusions
Despite similar findings across studies, there are a number of uncertainties about which dialysis modalities represent the most cost-effective options for patients at different points in the care pathway. Most studies included an incident patient cohort; however, in clinical practice, patients may switch between different treatment modalities over time according to their clinical need and personal circumstances.
Promoting health policies through financial incentives in renal care should reflect the cost-effectiveness of a comprehensive approach that considers different RRTs along the patient pathway; however, no such evidence is currently available.
Similar content being viewed by others
Introduction
Chronic kidney disease affects up to 16% of the adult population globally, and is associated with poor outcomes upon progression to end-stage renal disease (ESRD) [1]. Typical ESRD patients commencing renal replacement therapy (RRT) are inherently in rapidly declining health, are relatively old (median age of 64.0 years in the UK in 2018 [2]), have several common comorbid conditions, and span socioeconomic classes [3, 4]. While kidney transplant is regarded as the optimal RRT [5], donor availability and patient eligibility are limiting factors in practice [6,7,8]. For this reason, the majority of ESRD patients undergo dialysis. Figure 1 provides an overview of the current pathway for management of ESRD. The predominant dialysis modalities are haemodialysis (HD; which can be undertaken in-centre [ICHD] or at home [HHD]), and peritoneal dialysis (PD), which is generally undertaken at home [9,10,11]. These techniques, though all considered efficacious, are distinct in terms of their practical application. Eligibility for HHD and PD is dependent on the willingness and ability of patients to manage their own care, a suitable home environment in which to perform these procedures, and is subject to certain medical requirements [12, 13]. Patients may require a number of modality changes over their lifetime on RRT [14,15,16]. For example, many patients who receive PD may require a switch to a HD treatment modality after 2–3 years. Residual renal function is extremely important in PD-treated patients and needs to be monitored closely [17]. In addition, the peritoneal membrane is a living structure that is prone to alteration over time, for example due to dialysis fluid characteristics (e.g. pH, osmolarity, glucose degradation products, and bioincompatibility) and peritonitis, which lead to loss of the membrane properties that enable efficient treatment [18]. In clinical practice, that means that peritoneal membrane performances (water and solute mass permeability) should be monitored periodically to detect early deterioration [19]. In the case of dialysis inadequacy, a switch to another form of RRT should be considered [20]. Therefore, offering the best mode of dialysis at the right time for each patient is crucial [15, 16].
Appropriate decision-making is fundamental in renal care, for which effective implementation and continuation of care is crucial to prolonging survival and improving a patient’s quality of life. Furthermore, RRT is a significant burden on finite healthcare resources; across Europe RRT accounts for 2% of healthcare expenditure for 0.1% of the population, with an estimated total cost of €15 billion per year [21]. In general, economic evaluations are used to provide high-level predictions of whether an intervention may be of value to a system relative to established practice. Such analyses are often used to support decision-making at a national level, which subsequently influences local policies and individual decisions. However, the cost-effectiveness framework generally represents the use of an intervention at a particular point in care for the ‘average’ patient. As such, traditional methods are restrictive in accommodating the complexities of the actual life of a patient with a chronic disease such as ESRD.
National guidance and public health policies have emerged that recommend or incentivise specific dialysis modalities based upon conclusions of cost-effectiveness [22,23,24,25]. The aim of this analysis was to systematically identify and assess existing cost-effectiveness analyses of dialysis modalities and to understand whether the current published data reflect the complexities of the ESRD patient pathway.
Methods
A systematic literature review (SLR) was conducted to identify published cost-effectiveness studies comparing two or more distinct dialysis techniques in the management of ESRD. Since renal transplant is accepted to be the superior mode of RRT for clinically suitable patients [26], studies for which the objective was to assess the cost-effectiveness of transplant were excluded from this research; however, studies that considered transplant as one of several treatment options have been included (with results relevant to transplant not reported). The following databases were searched: Embase, MEDLINE, EBM Reviews and EconLit. The review was initially conducted between 2005 and March 2020 and was subsequently updated to capture the most recently published studies up to July 2021. The review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27]. The full search strategy is presented in Supplementary Information section S1 and S2.
Abstracts and full texts were screened by two reviewers based on the criteria described in Table 1. Studies that were not in English or for which no full text was available were excluded. Only studies published since and using direct costs reflective of 2005 onwards were considered, to ensure the relevancy of discussions to current practice. Studies that did not comprise a comparison of distinct dialysis methods (e.g. HD vs haemodiafiltration; continuous ambulatory peritoneal dialysis [CAPD] vs automated peritoneal dialysis [APD]) were also excluded. Therefore, the only comparisons considered in this analysis were: ICHD vs PD and/or HHD. Extraction of relevant variables and assessment of bias [28] for all included studies were performed by two reviewers, with any disagreements resolved by discussion and/or additional referees.
Results
Characteristics of included studies
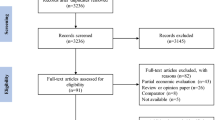
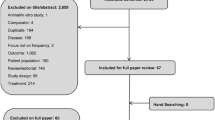
The initial search identified 2189 records and the update identified 189 records. In total, 19 publications comparing distinct modalities were eligible for inclusion (Fig. 2). The completed quality assessment of studies can be found in Supplementary Table 1.
Populations and interventions considered
A summary of the methodology of the included studies is presented in Table 2. All included studies considered adult patients undergoing dialysis for ESRD, while two studies restricted their patient populations to those > 60 years of age [29, 30]. The vast majority of studies (13/19) considered an incident population (i.e. patients who were starting dialysis for the first time) [29,30,31,32,33,34,35,36,37,38,39,40,41], while 6/19 studies did not specify, or considered a prevalent or mixed incident/prevalent population [42,43,44,45,46,47]. One study presented outcomes for subgroups of patients with or without diabetes [30]. In total, ten studies compared the cost-effectiveness of ICHD with PD only (or also with transplant, which is not a treatment option of consideration for this analysis) [30, 31, 33, 34, 37, 39, 40, 42, 45, 47], while a further three studies compared HD with PD only, but the location of HD (in centre or at home) was not reported [38, 41, 44]. Three studies compared ICHD with HHD only [32, 36, 43], two studies compared ICHD with HHD and PD [29, 35], while one study considered HD administered in-centre, at a satellite centre, as self-care or at home compared with PD [46]. Across the studies that considered HHD, the duration and frequency of administration varied from conventional (3 sessions × 4 h per week) to intensive home haemodialysis (iHHD; every other day, 5 times per week or nocturnal HD) [29, 32, 35, 36, 43, 46]. Eight studies looked at the potential impact of varying PD uptake rates from existing local levels [30, 34, 35, 37,38,39, 45]; one of these studies also looked at the impact of increasing HHD uptake from the existing treatment mix [35].
Model design, structure and perspective
Of the 19 included studies, 7 were conducted in Europe, 9 in Asia, 2 in Canada, and 1 in Australia (Table 2). The predominant model type was a Markov model, used in 16/19 of the included studies. The most common time horizon was a lifetime horizon (7/19 studies), with other model horizons considered including 5, 10, 12, and 15 years (Table 2). Three studies considered a range of time horizons [30, 37, 38]. Two studies conducted a cost-effectiveness analysis within a defined study period as opposed to extrapolating outcomes over a designated time horizon [33, 47]. Nine studies were conducted from a payer/health service perspective [32, 34, 35, 37, 40,41,42,43, 45], six from a societal perspective [30, 31, 36, 38, 44, 46], and two included both a payer and societal perspective [29, 39]. There was considerable variation in discount rates used across studies (1.5–6.0%), in accordance with local recommendations. Treatment switching to another modality was permitted in all but four studies [30, 33, 42, 47].
Data inputs
The most common clinical data inputs were survival/mortality data and treatment switching, which were included in the majority of studies (Table 2). Sources of such data typically included registries and published estimates. One study included complications associated with dialysis [31] and three included data on hospitalisations [32, 37, 43]. A wide range of utility values were utilised across the studies. Utility values applied ranged from 0.54 to 0.85 for ICHD, 0.54 to 0.75 for HHD, and 0.54 to 0.905 for PD (Table 2). Five studies applied a constant utility value across each dialysis modality [35, 38, 40, 41, 46]. Only one study applied a disutility for complications that led to treatment switching [31], and one study applied disutilities for the occurrence of certain adverse events [46]. All studies included direct costs of dialysis treatment; however, the extent of what elements were covered varied somewhat between studies. Most (13/19) studies incorporated some form of non-medical direct costs or indirect costs, but these were generally not comprehensive.
Study results
A summary of the results and reported limitations of each study is presented in Table 3. Across the 15 studies that compared ICHD/HHD with PD, PD was consistently reported to be the most cost-effective intervention. Similarly, across the 5 studies that compared HHD with ICHD, HHD was consistently reported to be the most cost-effective intervention, regardless of the frequency or duration of HHD (conventional or intensive) [32, 35, 36, 43, 46]. Only two studies assessed cost-effectiveness of HHD and PD and the results were mixed, with one study reporting that HHD was cost-effective when compared with PD [29], and the other reporting that HHD was not cost-effective when compared with PD [46].
Study limitations reported by the authors included a lack of good quality clinical data [31, 43] and utility data [32, 34, 36, 43], and a lack of adequate cost information [46]. A number of studies reported a lack of consideration of some elements, which may be of importance in the overall value of the treatment, such as hospitalisations, complications, training and indirect costs, as limitations. Some studies reported concerns over applicability of their findings to a wider geographical area [29, 31, 39].
Discussion
The majority of the studies identified in the SLR compared ICHD with PD, as these are the most common two treatment modalities used. Fewer studies included HHD as a modality option, most probably due to the relatively lower preference of this modality in recent years, and there was limited evidence comparing the cost-effectiveness of HHD versus PD. There remains to be a number of uncertainties surrounding which dialysis modalities represent the most cost-effective options for patients at different points in the care pathway.
There was considerable heterogeneity between the included studies across multiple aspects of the methodology, including time horizon, discounting, utility values, sources of clinical and economic data, and extent of clinical and economic elements included, which may have an impact on the outcomes of the analyses. Furthermore, studies were conducted from a wide range of country perspectives, where models of health care, availability of dialysis modalities and costs associated with providing health services may vary substantially. Studies were also conducted using a range of cost reference years and currencies. Taking all these factors into account, it is therefore difficult to compare studies with each other. A number of studies highlighted the lack of good quality clinical and utility data [31, 32, 34, 36, 43] and many also noted that their findings may be limited in their applicability to wider geographical settings due to the local nature of the clinical and economic inputs that were used [29, 31, 39].
Quality assessment of the included economic evaluations (provided in Supplementary Table 1) revealed that in general, the studies had well defined objectives, study design, and data collection methods. However, key modelling decisions (in particular, choice of type of economic evaluation, choice of model type, choice of discount rate and choice of variables for sensitivity analysis) were not consistently justified. Further, while results were generally clearly reported and included incremental analyses, there was variability in the extent to which individual caveats were discussed and issues relating to the generalisability of results were not often addressed.
Whilst in clinical practice, patients may switch between different treatment modalities over time according to their clinical need and personal circumstances [14,15,16], none of the included studies accounted for this. Most studies were cohort-level simulations representing treatments at a particular point in time, with the majority of studies considering incident patients at the start of their dialysis treatment journey, and none accounted for the fact that PD is likely to be a treatment option for a limited period of time for many patients [20, 48]. Although switching treatments was often permitted within models, no utility decrements associated with the need for a treatment switch, or additional costs associated with switching treatments were considered. Furthermore, any treatment switching effects were incorporated into the costs and outcomes for the initial treatment allocation. No studies investigated the cost-effectiveness of a switch to a different treatment modality.
Few studies noted the applicability of their results to the individual as a limitation; however, Treharne et al. (2014) highlighted that their findings concerning the cost-effectiveness of PD relative to ICHD would only be applicable to those patients who were willing and able to perform PD [37]. The same rationale also applies to HHD [13]. No studies highlighted the importance of patient choice or education in optimising clinical, economic and societal outcomes.
Previous reviews of dialysis have highlighted that there is no best dialysis modality for a patient, but rather a combination of different modalities applied at the right time for the right patient, to create an optimal treatment pathway [15, 16]. The cost-effectiveness of different modalities is therefore likely to vary over the disease course and depending on individual patient characteristics.
National guidance and public health policies have emerged that recommend or incentivise specific dialysis modalities based upon conclusions of cost-effectiveness [22,23,24,25]. A lack of clarity and education regarding the initiation of dialysis [49, 50] remains, so national policies may be adopted without proper consideration of an individual’s needs, resulting in suboptimal care. Financial incentives for specific modalities would place a hurdle to initiating other modalities at the clinician level, precluding consideration of patient choice and circumstances, and it has been reported that where there is little or no reimbursement of a modality, uptake can be low [51]. It has been noted that strong financial incentives for clinical outcomes risk undermining valued aspects of the service user–provider relationship [52]. The National Institute for Health and Care Excellence (NICE) guidelines for RRT in the UK state that the decision to start dialysis should be made jointly by the person (or, where appropriate, their family members or carers) and their healthcare team [49]. Shared decision-making between people and clinicians about their care leads to more realistic expectations, a better match between individuals’ values and treatment choices, and fewer unnecessary interventions [52, 53].
Decision-making within renal care should be holistic and comprehensive so that health outcomes are optimised for each patient and resources are allocated appropriately. Future work in this area should consider a pathway-based cost study, mapping costs to data from large care networks to more accurately reflect resource use associated with renal care.
Conclusions
Dialysis is a life-saving intervention, but a patient’s care pathway is likely to evolve over time due to a changing lifestyle, increasing age and declining health. Switching between dialysis modalities is often essential. The cost-effectiveness of any intervention is reliant on its effectiveness, which, in the case of dialysis, is inherently linked with patient acceptability of and suitability for a particular modality. Estimates of cost-effectiveness for a particular dialysis modality at a specific point in time should be considered within the context of the holistic ESRD pathway. Each dialysis method is distinct and may be applicable to different points in care; therefore, modalities should be considered complementary and not competitively. Personalised care, including offering the right modality to the right patient at the right time, will maximise patient outcomes and minimise expenditure.
Availability of data and material
Not applicable.
References
Stringer, S., et al.: The natural history of, and risk factors for, progressive chronic kidney disease (CKD): the Renal Impairment in Secondary care (RIISC) study; rationale and protocol. BMC Nephrol. 14, 95–95 (2013)
The Renal Association UK Renal Registry: 22nd Annual Report. (2018) 04/08/2020; Available from: https://www.renalreg.org/wp-content/uploads/2020/07/22nd_UKRR_ANNUAL_REPORT_FULL.pdf
Gomez, A.T., et al.: Comorbidity burden at dialysis initiation and mortality: a cohort study. Can. J. Kidney Health Dis. 2, 34–34 (2015)
Crews, D.C., et al.: Low income, community poverty and risk of end stage renal disease. BMC Nephrol. 15(1), 192 (2014)
The Renal Association: Assessment of the potential kidney transplant recipient, 5th edn. The Renal Association (2011)
NHS Choices Waiting list - kidney transplant. (2015)
Deutsche Stiftung Organtransplantation. Warteliste und Vermittlung (Waiting list and mediation) September 2018]; Available from: https://www.dso.de/organspende-und-transplantation/warteliste-und-vermittlung.html
MedTech Europe. Improving dialysis for patients and health systems in community and home care. Available from: https://www.medtecheurope.org/resource-library/improving-dialysis-for-patients-and-health-systems-in-community-and-home-care/ (2015)
Levin, A., Stevens, P.E.: Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85(1), 49–61 (2014)
Saggi, S.J., et al.: Considerations in the optimal preparation of patients for dialysis. Nat Rev Nephrol 8(7), 381–389 (2012)
Kidney Health New Zealand. Conservative treatment September 2018]; Available from: https://www.health.govt.nz/system/files/documents/topic_sheets/conservative-treatment.pdf
Blake, P.G., Quinn, R.R., Oliver, M.J.: Peritoneal dialysis and the process of modality selection. Perit Dial Int 33(3), 233–241 (2013)
Rioux, J.P., et al.: Patient selection and training for home hemodialysis. Hemodial Int 19(1), S71–S79 (2015)
Covic, A., et al.: Educating end-stage renal disease patients on dialysis modality selection: clinical advice from the European Renal Best Practice (ERBP) Advisory Board. Nephrol Dial Transplant 25(6), 1757–1759 (2010)
Imbeault, B., Nadeau-Fredette, A.C.: Optimization of dialysis modality transitions for improved patient care. Can J Kidney Health Dis 6, 2054358119882664 (2019)
Lambie, M., Davies, S.J.: Transition between home dialysis modalities: another piece in the jigsaw of the integrated care pathway. Nephrol Dial Transplant 30(11), 1781–1783 (2015)
Diaz-Buxo, J.A., White, S.A., Himmele, R.: The importance of residual renal function in peritoneal dialysis patients. Adv Perit Dial 29, 19–24 (2013)
Salzer, W.L.: Peritoneal dialysis-related peritonitis: challenges and solutions. Int J Nephrol Renovasc Dis 11, 173–186 (2018)
Struijk, D.G.: Monitoring of the peritoneal membrane NDT Plus. Clin Kidney J 1(4), 29–35 (2008)
Mayo Clinic. Peritoneal dialysis. 2021 July 2021]; Available from: https://www.mayoclinic.org/tests-procedures/peritoneal-dialysis/about/pac-20384725
European Kidney Health Alliance. Recommendations for Sustainable Kidney Care. 2015 July 2021]; Available from: http://ekha.eu/wp-content/uploads/2016/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf
Hager, D., Ferguson, T.W., Komenda, P.: Cost controversies of a “Home Dialysis First” Policy. Can J Kidney Health Dis. 6, 2054358119871541–2054358119871541 (2019)
Teerawattananon, Y., et al.: How to meet the demand for good quality renal dialysis as part of universal health coverage in resource-limited settings? Health Res Policy Syst 14, 21 (2016)
Teerawattananon, Y., Mugford, M., Tangcharoensathien, V.: Economic evaluation of palliative management versus peritoneal dialysis and haemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value in Health 10, 61–72 (2007)
Li, P.K., Chow, K.M.: The cost barrier to peritoneal dialysis in the developing world–an Asian perspective. Perit Dial Int 21(3), S307–S313 (2001)
NHS Kidney Care, Transplant first: timely listing for kidney transplantation. Available from: https://www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Transplant-First-Timely-Listing-for-Kidney-Transplantation.pdf (2013)
Page, M.J., et al.: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021)
Drummond, M.F., Jefferson, T.O.: Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 313(7052), 275–283 (1996)
Wong, C.K.H., et al.: Lifetime cost-effectiveness analysis of first-line dialysis modalities for patients with end-stage renal disease under peritoneal dialysis first policy. BMC Nephrol 21(1), 42 (2020)
Yang, F., Lau, T., Luo, N.: Cost-effectiveness of haemodialysis and peritoneal dialysis for patients with end-stage renal disease in Singapore. Nephrology (Carlton) 21(8), 669–677 (2016)
Afiatin, et al.: Economic evaluation of policy options for dialysis in end-stage renal disease patients under the universal health coverage in Indonesia. PLoS One 12(5), 0177436 (2017)
Beby, A.T., et al.: Cost-effectiveness of high dose hemodialysis in comparison to conventional in-center hemodialysis in the Netherlands. Adv Ther 33(11), 2032–2048 (2016)
Chang, Y.T., et al.: Cost-effectiveness of hemodialysis and peritoneal dialysis: a national cohort study with 14 years follow-up and matched for comorbidities and propensity score. Sci Rep 6, 30266 (2016)
Haller, M., et al.: Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant 26(9), 2988–2995 (2011)
Howard, K., et al.: The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology (Carlton) 14(1), 123–132 (2009)
Klarenbach, S., et al.: Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. J Am Soc Nephrol 25(3), 587–594 (2014)
Treharne, C., et al.: Peritoneal dialysis and in-centre haemodialysis: a cost–utility analysis from a UK payer perspective. Appl Health Econ Health Policy 12(4), 409–420 (2014)
Villa, G., et al.: Cost-effectiveness analysis of the Spanish renal replacement therapy program. Perit Dial Int 32(2), 192–199 (2012)
Bayani, D.B.S., et al.: Filtering for the best policy: An economic evaluation of policy options for kidney replacement coverage in the Philippines. Nephrology (Carlton) 26(2), 170–177 (2021)
Ferguson, T.W., et al.: Cost-utility of dialysis in Canada: hemodialysis, peritoneal dialysis, and nondialysis treatment of kidney failure. Kidney Med 3(1), 20-30.e1 (2021)
Yang, F., et al.: Cost-effectiveness analysis of renal replacement therapy strategies in Guangzhou city, southern China. BMJ Open 11(2), e039653 (2021)
Kontodimopoulos, N., Niakas, D.: An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy 86(1), 85–96 (2008)
Liu, F.X., et al.: High-dose hemodialysis versus conventional in-center hemodialysis: a cost–utility analysis from a UK payer perspective. Value Health 18(1), 17–24 (2015)
Moradpour, A., Hadian, M., Tavakkoli, M.: Economic evaluation of end stage renal disease treatments in Iran. Clin Epidemiol Glob Health 8(1), 199–204 (2020)
Surendra, N.K., et al.: Cost utility analysis of end stage renal disease treatment in ministry of health dialysis centres, Malaysia: hemodialysis versus continuous ambulatory peritoneal dialysis. PLoS ONE 14(10), e0218422 (2019)
Pike, E., et al.: More use of peritoneal dialysis gives significant savings: a systematic review and health economic decision model. J Clin Med Res 9(2), 104–116 (2017)
Wu, H., et al.: Economic burden and cost–utility analysis of three renal replacement therapies in ESRD patients from Yunnan Province, China. Int Urol Nephrol 52(3), 573–579 (2020)
Rydell, H., et al.: Fewer hospitalizations and prolonged technique survival with home hemodialysis- a matched cohort study from the Swedish renal registry. BMC Nephrol 20(1), 480 (2019)
National Institute for Health and Care Excellence. NG107: Renal replacement therapy and conservative management. 2018 27/08/2020]; Available from: https://www.nice.org.uk/guidance/NG107
CADTH (Canadian Agency for Drugs and Technologies in Health) Dialysis modalities for the treatment of end-stage kidney disease: a health technology assessment. Optimal Use Report 6(2b), 14–15 (2017)
Just, P.M., et al.: Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 23(7), 2365–2373 (2008)
Systems, A.-C.: Evidence, Strategies and Challenges. Cambridge University Press, Cambridge (2020)
NHS England. What is personalised care? 2020 04/08/2020]; Available from: https://www.england.nhs.uk/personalisedcare/what-is-personalised-care/
Funding
This study was funded by Fresenius Medical Care.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The systematic literature review was conducted by SM and HS. The first draft of the manuscript was written by HS and all authors commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Financial interests: EB, DK, FK, SB, JP and CA are employees of Fresenius Medical Care, who funded the manuscript. SM and HS are employees of Mtech Access, an independent market access consultancy, who were commissioned by Fresenius Medical Care to develop the systematic review and manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busink, E., Kendzia, D., Kircelli, F. et al. A systematic review of the cost-effectiveness of renal replacement therapies, and consequences for decision-making in the end-stage renal disease treatment pathway. Eur J Health Econ 24, 377–392 (2023). https://doi.org/10.1007/s10198-022-01478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-022-01478-2
Keywords
- Systematic review
- Renal replacement therapy
- Patient pathway management
- Patient choice
- Economic evaluation
- Healthcare policy