Abstract
Understanding the drivers of food web community structure is a fundamental goal in ecology. While studies indicate that many coral reef predators depend on pelagic subsidies, the mechanism through which this occurs remains elusive. As many of these species are important fishery targets, a better understanding of their trophodynamics is needed. To address these gaps, we employed a comprehensive structural equation modelling approach using extensive surveys of the reef community to explore relationships between groupers and snappers, their prey, and the surrounding habitat in an oceanic coral reef system. There were significant positive relationships between site-attached and transient planktivores and grouper and snapper biomass, respectively, indicating that pelagic subsidies are transferred to upper trophic levels through planktivores. Contrary to previous studies, habitat complexity and depth were not important for predators or prey. Instead, corallivores and site-attached and transient planktivores were primarily associated with live habitat and coral cover. This indicates that a decline in coral cover could have severe direct and indirect impacts on the structure and functioning of multiple levels of the reef food web. While pelagic reliance may suggest that predators are resilient to bleaching-related habitat loss, the associations of their planktivorous prey with live coral suggest that both benthic and pelagic pathways should be preserved for continued resilience of these food webs and their fisheries. By considering direct and indirect relationships, our study generated insights not only on the complex dynamics of coral reef ecosystems, but also on how they may respond to environmental change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding food web dynamics and the drivers of community structure is a central goal in ecology. However, food webs are intricate, featuring numerous interconnected species, making it challenging to unravel the trophic interactions that shape community composition (Polis and Strong 1996). While predator–prey relationships in marine systems have received considerable attention (Hobson 1979; Baum and Worm 2009; Allain et al. 2012; Mitchell and Harborne 2020; Mihalitsis et al. 2021), the driving forces behind predator distributions and the impacts of prey assemblages on predators often remain elusive. Investigating predator–prey distributions across spatial scales, while taking into consideration local resource availability and the structure of the underlying benthos, may offer insight into the biotic and abiotic factors that shape predator community structure (Beukers-Stewart et al. 2011; Sandom et al. 2013). This is important for understanding how these communities may respond to environmental change and for assessing the function and future resilience of diverse food webs.
On coral reefs, predators such as lutjanids and serranids play a crucial role in shaping prey communities (Boaden and Kingsford 2015), contributing to the integrity and resilience of the ecosystem (McCann et al. 2005; Rooney et al. 2006; Ceccarelli and Ayling 2010). They are also important target species for reef fisheries, providing food and livelihoods to millions globally (Sadovy 2005; FAO 2016). Stable isotope analysis, which traces energy flows through food webs, indicates that these predators rely heavily on planktonic production for sustaining their biomass in many oceanic and offshore systems (Frisch et al. 2014; Matley et al. 2017; Skinner et al. 2019, 2021). This linkage is likely established through feeding on planktivores (Matley et al. 2018; Skinner et al. 2019), a productive component of the reef fish biomass (Williams and Hatcher 1983; Moritz et al. 2017; Morais et al. 2021) that have exceptional diversity across the Indo-Pacific (Siqueira et al. 2021). Given the dominance of planktivores on the reef and the pelagic reliance of these fishery-target reef predators, it is likely that predator distributions are influenced by the availability of planktivorous prey. Disentangling these relationships, including the simultaneous influence of other biotic and abiotic factors, is crucial to better understand, and manage, their populations.
Predator–prey dynamics on coral reefs have provided inconsistent evidence for either top-down or bottom-up directionality. For example, there was a strong positive relationship between abundances of reef predators and their prey on the Great Barrier Reef (Stewart and Jones 2001; Beukers-Stewart et al. 2011; Desbiens et al. 2021) and prey biomass was an important driver of reef-associated shark abundances in the Chagos Archipelago (Tickler et al. 2017). In contrast, in a longer-term study in the US Virgin Islands, there was a negative correlation between predator abundance and the maximum number of co-occurring prey, with no overall relationship between predator and prey abundances (Hixon and Beets 1993). Reef predators are also undoubtedly influenced by their environment, with various abiotic factors such as depth, habitat complexity, and flow likely shaping relationships within these ecological groups (Desbiens et al. 2021). Habitat complexity serves as a refuge for prey, while simultaneously affecting predator growth rates and potentially altering food web dynamics (Graham and Nash 2013; Rogers et al. 2018). Moreover, species compositions of both predator and prey fish communities also exhibit changes with varying depths (Jankowski et al. 2015; Asher et al. 2017). However, interpreting the role that these variables play can be complicated due to their indirect nature, so they are often overlooked in traditional predator–prey models. Consequently, our understanding of how these variables interact, and which predominantly influences the biomass of these important fishery-target species, remains elusive.
Here, we set out to test whether fishery-target reef predators have coinciding spatial distributions with planktivores, while simultaneously considering how environmental factors might influence these relationships in a coral reef food web in the Maldives. The Maldives offers a unique opportunity to test these dynamics, as there is substantial evidence that these predators rely on planktonic subsides in this region (Skinner et al. 2019, 2021). Furthermore, as key targets, these species contribute considerably to the local reef fishery, for which there is a growing demand (Sattar et al. 2014; Yadav et al. 2021). Given the combined stress of increasing fishery exploitation and repeated bleaching events (Pisapia et al. 2019), a deeper understanding of the factors influencing fishery-target predators and the complex dynamics that structure these ecosystems is needed.
Materials and methods
Survey sites
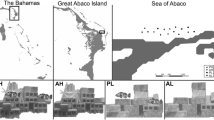
All fieldwork was conducted in North Malé Atoll, Maldives (N 04° 25′ 46.2″, E 73° 30′ 4.3″) between March and April 2018 (NE Monsoon). Ocean current flow direction varies with the monsoon; during the NE monsoon, productivity is higher on the west coast (Sasamal 2007). As the atoll is porous, consisting of outer edge reefs with deeper channels allowing an exchange of water between the open ocean and the inner atoll lagoon, planktonic resources are readily available throughout (Sasamal 2007; Skinner et al. 2019). Surveys were split between two regions: inner lagoonal reefs (hereafter “inner”), and outer edge reefs (hereafter “outer”). Inner reef sites are shallower (mean ± s.d. = 4.96 ± 0.84 m) than outer reef sites (mean ± s.d. = 10.31 ± 1.14 m).
Underwater visual census (UVC)
UVC was conducted at 40 sites, 20 in each atoll region (Fig. 1). At each site, two 30 × 5 m transects were randomly surveyed parallel to the forereef slope between 3 and 15 m. All transects were at least 10 m apart to ensure independence. The entire fish community was recorded to genus or species level, depending on their diet and body morphometries (Tables S1 and S2: full species list and classifications), and their size was recorded to the nearest centimetre (cm). When members of the same genus all had the same diet and a similar body shape (required for length–weight conversions) they were recorded to genus, but when diet or body shape differed within the genus, they were recorded to species. Blennies were recorded as two groups, sabretooth blennies and blennies, and gobies as reef or sand gobies (Kuiter 2014). For surveying, the fish community was split into larger, more mobile species (e.g. Acanthuridae, Lutjanidae, Lethrinidae) and smaller, cryptic site-attached species (e.g. Blenniidae, Gobiidae, Pomacanthidae). A first observer recorded all mobile fish while reeling out the transect, while a second observer came behind and swept each side of the transect for smaller, cryptic species. The substrate was also recorded every 50 cm along the transect. Habitat complexity was measured using a reef rugosity index by draping a fine-link chain along a 10 m section of the transect tape from 10 to 20 m and the place the chain reached was recorded. Reef rugosity was calculated using Eq. 1; all values are > 1, with larger values signifying a greater surface area and higher complexity, while smaller numbers signify a flatter, lower relief reef.
Fish classifications
This study aimed to explore site-level relationships between fishery-target predators, their prey, and the surrounding environment. As such, mobile and transient predator species (i.e. Aphareus furca, Aprion virescens, Caranx melampygus, Elagatis bipinnulata, and Gymnosarda unicolor) were excluded from further analysis. However, at the site-level, non-targeted predators might influence prey availability for target species. To check their influence, we explored the distributions of non-target predator species (i.e. Syngnathiformes Aulostomus chinensis and Fistularia commersonii, and lionfish Pterois antennata and P. volitans) that are not important to the Maldives reef fishery (Sattar et al. 2014) across the survey sites. Both Syngnathiformes and lionfish were present in exceptionally low numbers across all surveys: they occurred at only 5 sites in the inner atoll, and 3 and 9 outer atoll sites, respectively. Biomass contributions were also consistently low: each group contributed < 1% to total fish biomass across inner and outer atoll sites. As a result, they were not included in further analyses.
All remaining fish were categorised as prey into four feeding groups based on the literature and FishBase: benthic carnivores, corallivores, EAM feeders (Epilithic Algal Matrix, i.e. herbivores and detritivores), and planktivores (Table S1). Planktivores were further separated into two groups based on their habitat use: site-attached (e.g. Pseudanthias spp.) and transient (e.g. Caesionids). Any omnivores, including facultative corallivores, were excluded, as individuals feed across several potential energy pathways, obscuring linkages between predators and specific resource pools. Predator body size limits the size of the prey they can consume: Lutjanidae and Serranidae up to 50 cm generally consume prey that are < 20 cm (St John 1999; Dunic and Baum 2017), but predators can eat prey approximately half their own body length (Mihalitsis and Bellwood 2017). As such, when the largest predator was ≤ 40 cm (35 out of 40 sites), we excluded prey > 20 cm from those sites. However, at 5 out of the 40 sites, some individual predators were > 40 cm. For these sites, we constrained the size of the prey to 50% of the largest predator size. Finally, given their varied, and often opportunistic, diets, it was assumed that all prey from any of the feeding groups could be fed on by any of the predators (Shpigel and Fishelson 1989; St John 1999; Dierking et al. 2011; Barley et al. 2017).
The final analysis included predator and prey fish belonging to 25 families (Tables S1 and S2). All corallivores (n = 8 species from Chaetodontidae, Labridae, Monacanthidae) and fishery-target predators (n = 21 species from Lutjanidae, Serranidae) were identified to species. Transient planktivores were identified to genus (n = 2 from Caesionidae) or species (n = 4 from Acanthuridae), and site-attached planktivores to species (n = 14 from Acanthuridae, Balistidae, Chaetodontidae, Labridae, Microdesmidae, Pempheridae, Pomacentridae) and genus (n = 5 from Holocentridae, Pomacentridae, Serranidae). EAM feeders were either species (n = 15 from Acanthuridae, Pomacentridae) or genus (n = 7 from Pomacentridae, Scaridae, Siganidae), with Blennies (except for Sabretooth) included as a group also. Benthic carnivores were the largest group, identified to species (n = 10 from Balistidae, Chaetodontidae, Diodontidae, Lethrinidae, Pomacanthidae, Pomacentridae, Zanclidae), genus (n = 7 from Holocentridae, Labridae, Nemipteridae, Pinguipedidae), thicklip or maori wrasse (n = 4 genera from Labridae), other wrasse (n = 7 species and n = 3 genera from Labridae), family (Apogonidae and Mullidae), or Sabretooth Blennies.
Data analysis
Data were analysed in R statistical software version 4.2.1 (R Core Team 2023). Fish biomass was calculated using length–weight relationships from FishBase (Froese and Pauly 2023). At each site, benthic cover, prey, and predator biomass data were averaged across transects. Patterns in the benthic percentage cover data were explored using Principal Component Analysis (PCA) with Euclidean distance measures with the FactoMineR (Le et al. 2008) and FactoExtra packages (Kassambara and Mundt 2017). PCA standardises the data and generates a two-dimensional ordination which reduces dimensionality when using many quantitative variables (Jolliffe and Cadima 2016). Despite the potential for non-Euclidean distances in multivariate data analysis, our choice to use a PCA with Euclidean distances of the benthic cover data is justified by the shared characteristics of common features among sites, and the absence of a horseshoe effect, a phenomenon where the second axis of PCA represents a quadratic distortion of the first axis (Podani and Miklós 2002). The scores for the first two PC orthogonal axes for each site were extracted and used as explanatory variables for the benthic habitat in the subsequent modelling. The mean rugosity, depth, and biomass of each fish group at each site were plotted onto the extracted PC1 and PC2 coordinates of the benthic variables to visualise relationships in fish and benthic community structure. Differences in depth, rugosity, and fish group biomass between atoll regions were assessed using parametric t-tests for normal data and non-parametric Wilcoxon tests for non-normal data.
Structural equation modelling (SEM) and path analysis were used to explore all relationships. Unlike models which examine the impact of a limited number of variables on a single biological endpoint, such as abundance or biomass (Lefcheck and Freckleton 2016; Seibold et al. 2018; Bruder et al. 2019), SEMs incorporate multiple interrelated predictor and response variables, making them a useful tool for unravelling intricate systems with both direct and indirect linkages (Shipley 2002; Grace 2006; Grace et al. 2010). Hypothetical pathways between variables are identified a priori and are expressed in equation form, with response variables driven by one or multiple predictor variables. These response variables can become predictors for other variables, forming a sequence of causal relationships (Fan et al. 2016; Lefcheck and Freckleton 2016). Models are fit using maximum-likelihood estimation, which continually refines parameter estimates to minimise differences between the observed and expected variance–covariance matrices (Lefcheck and Freckleton 2016).
Here, a full conceptual model reflecting the structure of a coral reef food web was first developed to explore which biotic and abiotic variables might influence reef predator biomass (Fig. 2): 1) depth and atoll region (inner/outer) were considered predictors of the benthic habitat, 2) depth, benthic habitat, and atoll region of rugosity, 3) benthic habitat and rugosity of all prey fish groups, and 4) rugosity and the biomass of all prey fish groups of target predator biomass. By considering this web of relationships, SEMs provide a comprehensive framework to explore the underlying dynamics of complex coral reef ecosystems.
Conceptual pathway model of the biotic and abiotic variables influencing reef predator biomass. Fish silhouettes are from fishualize (Schiettekatte et al. 2019)
Before testing the SEM, data were inspected using pairwise plots and to assess collinearity between variables. All variables were checked for normality using a Shapiro–Wilks test and all fish biomass data and depth were log-transformed. Some variables remained non-normal after transformation. To account for this, a bootstrapping approach based on 1000 draws was used to estimate the model test-statistics and the standard errors for the SEM parameter estimates (Rosseel 2012). Using the lavaan R package (Rosseel 2012), the conceptual full model was fit with all predicted pathways. Following this, non-significant pathways were removed until the most parsimonious model was achieved. Standardised coefficients were used to assess the importance of predictor variable paths, as they can be used to compare variables on different scales (Kwan and Chan 2011). Model fit was assessed using the root mean square error of approximation (RMSEA), the comparative fit index (CFI), the Bayes Information Criterion (BIC), and the standard errors of the parameter estimates. Models with an RMSEA ≤ 0.08 (Browne and Cudeck 1992) and a CFI ≥ 0.90 (Hu and Bentler 1998) are considered a reasonable fit for SEMs, i.e. the model can reproduce the variance–covariance matrix of the data. The most parsimonious model had the greatest number of significant pathways, the lowest RMSEA and BIC, and the highest CFI.
Results
Benthic and fish community data
There was substantial separation in the benthic variables between atoll regions, and the outer sites were more clustered than inner sites (Fig. 3). The variables driving the outer sites were live coral, crustose coralline algae (CCA) and algae (i.e. combined turf and macroalgae), while the variables characterising the inner sites were sand, rock, and rubble. Sites were separated by live or abiotic substrate along PC1 and by coral or algal cover along PC2.
Outer sites were significantly deeper than those in the inner atoll (Fig. 4a; Table S3). There was no significant difference in rugosity between inner (mean ± s.d. = 1.53 ± 0.18) and outer sites (mean ± s.d. = 1.45 ± 0.10) (Table S3), with plots on the PC1 and PC2 coordinates revealing no clear patterns (Fig. 4b).
Biomass of EAM feeders and transient planktivores was significantly higher in the inner atoll (EAM feeder = 19.99 ± 5.73 g/m2; transient planktivores: 21.84 ± 19.50 g/m2) than in the outer atoll (EAM feeder = 16.48 ± 3.85 g/m2; transient planktivores = 19.13 ± 40.55 g/m2), while site-attached planktivores had a significantly higher biomass in the outer atoll (45.24 ± 31.72 g/m2) than in the inner atoll (17.25 ± 11.08 g/m2) (Table S3). There were no significant differences in the biomass of the other fish groups between inner and outer atoll regions. Grouper biomass (Figure S1a) was similarly distributed to the corallivores (Figure S1e) and site-attached planktivores (Figure S1g). Snapper biomass was low across all sites (Figure S1b, c), making it hard to identify trends with potential prey.
SEM model and pathways
Full model
The full (null) predictive model was a poor fit and did differ significantly from the observed data (Table 1). There were seven significant pathways: atoll region had a significant positive effect on PC1, PC2 significantly negatively influenced benthic carnivores, PC1 significantly positively influenced corallivores and site-attached planktivores, and significantly negatively influenced transient planktivores, site-attached planktivores significantly positively influenced groupers, and benthic carnivores significantly positively influenced snappers (Table 2; Figure S2). There were no significant covariances.
Most parsimonious model
All model statistics improved for the most parsimonious model indicating that it was a good fit, and it did not differ significantly from the observed data (Table 1). There were ten significant pathways in the parsimonious model, with all seven significant pathways from the full model retained (Fig. 5; Table 3). The pathways between atoll region and PC2, PC2 and EAM feeders, and transient planktivores and snappers were not significant in the full model but became so in the parsimonious model. The pathways between PC1 and depth to rugosity, and between PC2 and site-attached planktivores, were not significant, but their inclusion improved all the model fit statistics. Atoll region had a significant positive effect on PC1 and PC2, PC1 significantly positively influenced corallivores and site-attached planktivores and negatively influenced transient planktivores. Benthic carnivores and EAM feeders were significantly negatively influenced by PC2. Groupers were significantly positively influenced by site-attached planktivores, and snappers positively by both transient planktivores and benthic carnivores. There were no significant covariances.
The parsimonious model exploring the abiotic and biotic drivers of reef predator biomass. Single arrows indicate causal paths with standardised path coefficients. Thick arrows indicate significant relationships with stars showing the level (* = p < 0.05, ** = p < 0.01, *** = P < 0.001). Thin arrows signify non-significance. Fish silhouettes are from fishualize (Schiettekatte et al. 2019)
Discussion
Understanding the factors influencing reef fish biomass, especially fishery-target species that support coastal communities globally (FAO 2016), is crucial for assessing their ability to withstand future environmental changes. In this study, a comprehensive structural equation modelling (SEM) approach provided a unique perspective on the organisation of the coral reef food web in an oceanic atoll system. Unlike traditional linear modelling techniques, this approach allows for a thorough exploration of the often indirect relationships in the food web, yet to date, they have been infrequently used in marine systems (but see Casey et al. 2017; Morais and Bellwood 2019; Desbiens et al. 2021; Brown et al. 2023). The final SEM revealed significant connections among predators, their prey, and the habitat, demonstrating that energy flows from the base of the food web to upper trophic levels through their prey. This underscores the degree of interconnectedness in these ecosystems, demonstrating which trophic pathways connect predators with basal production via their prey (Lynam et al. 2017).
As expected, there were positive relationships between planktivores and both groupers and snappers. This is contrary to top-down predator control theory which, despite being common in food web ecology, is not consistent in the marine environment (Baum and Worm 2009). While reef predator pelagic reliance is known (Frisch et al. 2014; Ali et al. 2016; Matley et al. 2017; Skinner et al. 2019, 2021), the mechanism through which this ecological linkage occurs has remained unclear. The direct and significant relationships identified here reinforce the hypothesis that pelagic subsidies are transferred to predators through planktivores, fuelling the atoll food web regardless of habitat complexity (Morais and Bellwood 2019). By feeding on incoming plankton, and in turn supporting predators, planktivores play a fundamental role linking bottom-up and top-down processes (Lynam et al. 2017).
While groupers were positively associated with site-attached planktivores, snappers were associated with transient planktivores, but also benthic carnivores. This corroborates grouper and snapper behaviour, and their prey and habitat preferences. Groupers are relatively site-attached ambush predators (Shpigel and Fishelson 1989; Farmer and Ault 2011; Karkarey et al. 2017; Mihalitsis and Bellwood 2019). They mostly strike from below, capturing deep-bodied, social planktivores (Mihalitsis and Bellwood 2021; Mihalitsis et al. 2021) such as Pomacentrids (Matley et al. 2018) and elongated Clupeids (Ali et al. 2016). Conversely, snappers opportunistically feed on organisms in the water column or on benthic crustaceans (Grimes 1979; McCawley et al. 2006; Bacheler et al. 2021); stomach contents analyses reveal a diverse array of prey, ranging from reef-associated fish to various crustaceans (Hobson 1974; Kulbicki et al. 2005; Martínez-Juárez et al. 2024). Associations between snappers and benthic carnivores may therefore reflect both direct and indirect pathways: while snappers may prey upon smaller benthic carnivores (e.g. Blenniidae or some Labridae), they likely also compete with larger benthic carnivores (e.g. Mullidae) for invertebrate prey. Ultimately, disentangling these dynamics requires information on predator gape size, which constrains predator resource use based on prey body depth (Mihalitsis and Bellwood 2017). Here, in the absence of prey depth and predator gape size, we constrained the available prey at each site based on predator and prey body length (Mihalitsis and Bellwood 2017), however additional insight into these feeding dynamics might be achieved by incorporating individual predator gape size in future work. Snappers strike their prey horizontally from larger distances (Mihalitsis and Bellwood 2021) and often leave the reef at night to feed. Consequently, water column characteristics might also be responsible for the associations between transient planktivores and snappers, as flow is positively associated with planktivores and predatory reef sharks on the Great Barrier Reef (Desbiens et al. 2021). Although flow was not measured in this study, the current patterns in the Maldives fluctuate consistently with the monsoon; during the NE Monsoon currents flow from east to west (Sasamal 2007). Nevertheless, future studies would benefit from measuring small-scale fluctuations in flow to further disentangle any effects on the reef community.
The primary factor influencing the prey groups was not rugosity or depth, but the benthic substrate. Corallivores and site-attached planktivores were driven by live substrate, and site-attached planktivores also by coral-dominated substrate. Obligate corallivores are strongly associated with the habitat (Boaden and Kingsford 2015) and live coral cover, their primary food source (Bell and Galzin 1984; Bouchon-Navaro and Bouchon 1989; Darling et al. 2017). Indeed, even when reef structural complexity remains intact (Benkwitt et al. 2019), there are significant declines in corallivore populations following mass coral bleaching events (Wilson et al. 2006; Rice et al. 2019). Food availability is thus more critical for sustaining corallivore populations than the physical habitat structure alone. While there was no significant relationship between corallivores and groupers or snappers, they did covary (though not significantly). Groupers and snappers prey on a range of reef-associated fish species, but corallivores are not a major component of their diet (Dierking et al. 2011; Ali et al. 2016). Instead, despite having the lowest overall biomass among the surveyed groups, corallivores are likely a proxy for a healthier reef state characterised by a higher percentage of live coral, thereby supporting a greater biomass of predators. Indeed, large predators (but also small and large planktivores) are positively correlated with coral cover (Russ et al. 2017, 2021) and reef predator populations decline with decreasing coral cover (Sandin et al. 2008). Clearly, successive bleaching events, which are already common in the Maldives (Pisapia et al. 2019), will have a severe impact not just on corallivores but also on other groups. In contrast to the site-attached planktivores, transient planktivores were significantly associated with abiotic substrate. Other studies have identified positive associations between coral cover and Caesionids (Russ et al. 2017, 2021), likely as they use the reef as a sleeping habitat at night. Conducting our surveys later in the day, or at dusk, and better capturing the transient and spatio-temporally variable nature of their population through increased survey replication may have better explained their relationships with the benthic habitat.
EAM feeders were strongly associated with algal cover. Although turf and macroalgae were combined into one group for the analyses, macroalgae cover was low across the atoll (inner: 4.02% ± 5.57; outer = 2.01% ± 3.07) so this relationship was likely driven by turf algae cover (inner: 21.72% ± 11.85; outer = 33.20% ± 10.20). Positive relationships between grazers and their preferred algal resources are common, as fish tend to aggregate in zones of highest food availability (Williams and Polunin 2001; Russ 2003; Oakley-Cogan et al. 2020). However, in areas with higher wave exposure, there is a decrease in herbivore biomass despite increases in algal turf (Williams et al. 2013; Heenan et al. 2016), suggesting there is a wave exposure threshold beyond which positive relationships between herbivores and algae are decoupled. Benthic carnivores were significantly associated with algal/rubble substrate. Although invertebrates, which serve as prey for benthic carnivores, were not recorded during the surveys, rubble habitat was. Rubble can serve as a proxy for invertebrate density (Kramer et al. 2015; Desbiens et al. 2021) so aspects of these dynamics were incorporated, but rubble beds vary in their complexity and overall function for cryptobenthic organisms (Wolfe et al. 2021, 2023; Kenyon et al. 2023). Future studies would therefore benefit from including measures of all potential food items to disentangle these relationships at finer scales.
At the base of the food web, benthic cover at inner and outer atoll sites was almost entirely distinct from one another. While differences in habitat characteristics between inner and outer reefs vary among atolls (Cowburn et al. 2019), this is consistent with other studies that observe differences in reef habitat between lagoons and outer edge reefs (Brown et al. 2018), particularly in the Maldives (Morri et al. 2015; Pisapia et al. 2016). Sites in the inner atoll had more soft-bottom habitat, characterised by sand, rock, and rubble, while outer sites had almost double the live coral cover (inner = 15.61% ± 10.43; outer = 28.24% ± 6.31). This disparity is likely also related to the degree of exposure; outer sites are closer to open ocean currents that could help alleviate temperature stress, reducing the level of bleaching-induced coral morality (Safaie et al. 2018; Pisapia et al. 2019). However, increased wave exposure and depth on outer sites can also reduce coral recovery potential and growth compared to shallow sheltered reefs (Cowburn et al. 2019).
In contrast to previous studies (Graham and Nash 2013; Rogers et al. 2014; Jankowski et al. 2015; Darling et al. 2017; Ferrari et al. 2017; Beese et al. 2023), depth did not significantly influence any of the groups in the final model. Furthermore, while the inclusion of rugosity did improve model fit, it was not significant and did not influence the prey or predator groups. This could be related to the atoll considered. For example, here, all transects were conducted on the upper forereef slope within a relatively narrow depth range (3 – 15 m), so potential changes in community biomass at greater depths were not observed. Additionally, mean rugosity was similar between inner and outer atoll with a relatively narrow range (inner 1.12 – 1.94; outer 1.26 – 1.71). This lack of variation may reflect a flattening of the reefs following the 2016 bleaching event (Newman et al., 2015; Pisapia et al. 2019), although differences in rugosity across sites likely occurs across other atolls in the region, and globally. As such, it is also possible that variability in rugosity and depth between the inner and outer sites was captured in the atoll region variable; it was important in explaining differences between sites while depth and rugosity were not. Likewise, the rugosity variable may not provide additional information beyond the benthic principal component axes. For instance, indicators such as live coral cover or rubble, which were interpreted in terms of food availability, offer habitat for refuge as well. Consequently, the relationship between benthic habitat and the prey community may also include the influence of refuge availability. To enhance future research and better capture the relationships between the reef community and habitat complexity, it would serve to incorporate additional measures of structural complexity (e.g. fractal dimension and substrate height) (Torres-Pulliza et al. 2020) and refuge availability (e.g. hole size and abundance) into models, while surveying transects across a wider range of sites.
Although extensive underwater visual surveys were conducted and SEMs offer a useful modelling tool for gaining insights into these dynamics, it is impossible to capture all predator–prey-habitat relationships comprehensively. Arriving at robust solutions with a SEM also requires a substantial amount of data, as the model evaluates multiple hypotheses simultaneously (Grace 2006; Lefcheck and Freckleton 2016). While the final model achieved a good fit, a larger dataset with the inclusion of additional parameters, such as predator gape size, invertebrate biomass, primary production, or temperature, could provide further insights into the strengths of the predator–prey-habitat relationships on these reefs. There may also be other considerations for studies in different locations. For example, non-targeted predators could influence prey availability for target species, but there were few recorded across our study sites. Removal of target species through fishing would also likely change these associations, impacting reef fish densities and species richness by releasing prey from predation pressure (Boaden and Kingsford 2015; Hensel et al. 2019). Here, however, fishing pressure was assumed to be consistent across our sites. While resort islands in the Maldives often restrict fishing on their house reefs (acting as de facto protected areas), this has little to no effect on commercial fish species (Moritz et al. 2017; Chang 2020), likely as fishing activities are simply displaced to nearby reefs. Furthermore, only two of our forty survey sites were on resort house reefs, and fishing pressure is prevalent across inner and outer atoll regions (Sattar et al. 2012; Skinner, C. pers. obs.). Other studies should consider fishing pressure as a potential factor when exploring relationships such as these, however, particularly as removal of target species may impact the trophodynamics of the system. Other caveats to the SEM approach are, for example, that bi-directional paths cannot be analysed, making it challenging to incorporate feedback loops (despite their potential importance for ecosystem stability e.g. Neutel and Thorne 2014), and that the identified relationships may not demonstrate cause and effect, but rather associations and correlations (Fan et al. 2016). Nevertheless, the final model provides a fresh perspective on the factors shaping reef fish communities and confirms that food availability, rather than abiotic variables such as rugosity or depth, primarily structures predator–prey-habitat relationships in this system.
This analysis underscores the importance of planktivore biomass in supporting fishery-target reef predator communities (Morais and Bellwood 2019; Skinner et al. 2019, 2021; Morais et al. 2021). Reef fisheries in the Maldives provide an important source of food to both tourists and locals, with a rise in catches evidence of a growing demand for these resources (Sattar et al. 2014; Yadav et al. 2021). While the planktonic reliance of these predators might initially suggest resilience to bleaching-related habitat losses, their vulnerability becomes apparent when considering the dependence of their key planktivorous prey on live coral habitat. Moreover, in addition to impacts of habitat loss, many of these planktivores, including transient Caesionids but also site-attached Pomacentrids, are also targets of the tuna bait fishery (Anderson 1997). Increasing exploitation of these planktivores will not only impact the structure and functioning of the reef food web but will jeopardize the livelihoods of those depending on these reefs. Clearly, the dynamics structuring fishery-target predators in this system are complex. Whole-of-ecosystem-based management practices are required to effectively safeguard both the benthic and pelagic pathways supporting these ecosystems to ensure their long-term sustainability.
Data availability
The data in this paper and the code used to generate analyses will be made available on a public online repository if the paper is accepted.
References
Ali MKH, Belluscio A, Ventura D, Ardizzone G (2016) Feeding ecology of some fish species occurring in artisanal fishery of Socotra Island (Yemen). Mar Pollut Bull 105:613–628
Allain V, Fernandez E, Hoyle S, Caillot S, Jurado-Molina J, Andrefouet S, Nicol S (2012) Interaction between coastal and oceanic ecosystems of the western and central Pacific Ocean through predator- prey relationship studies. PLoS ONE 7:e36701
Anderson RC (1997) The Maldivian tuna livebait fishery–status and trends. In: Nickerson DJ, Maniku MH (eds) Report and Proceedings of the Maldives/FAO National Workshop on Integrated Reef Resources Management in the Maldives March 1996 BOBP/REP/76; , Malé, Maldives: BOBP/REP/76 p. 69–92.
Asher J, Williams ID, Harvey ES (2017) An assessment of mobile predator populations along shallow and mesophotic depth gradients in the Hawaiian Archipelago. Sci Rep 7:3905
Bacheler NM, Shertzer KW, Runde BJ, Rudershausen PJ, Buckel JA (2021) Environmental conditions, diel period, and fish size influence the horizontal and vertical movements of red snapper. Sci Rep 11:9580
Barley SC, Meekan MG, Meeuwig JJ (2017) Diet and condition of mesopredators on coral reefs in relation to shark abundance. PLoS ONE 12:e0165113
Baum JK, Worm B (2009) Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol 78:699–714
Beese CM, Mumby PJ, Rogers A (2023) Small-scale habitat complexity preserves ecosystem services on coral reefs. J Appl Ecol 60(9):1854–1867
Bell K, Galzin R (1984) Influence of live coral cover on coral-reef fish communities. Mar Ecol Prog Ser 15:265–274
Benkwitt CE, Wilson SK, Graham NAJ (2019) Seabird nutrient subsidies alter patterns of algal abundance and fish biomass on coral reefs following a bleaching event. Glob Change Biol 25:2619–2632
Beukers-Stewart BD, Beukers-Stewart JS, Jones GP (2011) Behavioural and developmental responses of predatory coral reef fish to variation in the abundance of prey. Coral Reefs 30:855–864
Boaden AE, Kingsford MJ (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6:1–33
Bouchon-Navaro Y, Bouchon C (1989) Correlations between chaetodontid fishes and coral communities of the Gulf of Aqaba (Red Sea). Environ Biol Fishes 25:47–60
Brown KT, Bender-Champ D, Kubicek A, Van der Zande R, Achlatis M, Hoegh-Guldberg O, Dove SG (2018) The dynamics of coral-algal interactions in space and time on the southern Great Barrier Reef. Front Marine Sci 24(5):181
Brown C, Ahmadia GN, Andradi-Brown DA, Arafeh-Dalmau N, Buelow CA, Campbell MD, Edgar GJ, Geldmann J, Gill D, Stuart-Smith RD (2023) Entry fees enhance marine protected area management and outcomes. Biol Cons 283:110105
Browne MW, Cudeck R (1992) Alternative ways of assessing model fit. Sociol Methods Res 21:230–258
Bruder A, Frainer A, Rota T, Primicerio R (2019) The importance of ecological networks in multiple-stressor research and management. Front Environ Sci 7(7):59
Casey JM, Baird AH, Brandl SJ, Hoogenboom MO, Rizzari JR, Frisch AJ, Mirbach CE, Connolly SR (2017) A test of trophic cascade theory: fish and benthic assemblages across a predator density gradient on coral reefs. Oecologia 183:161–175
Ceccarelli D, Ayling T (2010) Role, importance and vulnerability of top predators on the Great Barrier Reef - A review. Great Barrier Reef Marine Park Authority
Chang S (2020) Exploring the Spatial Relationships between Resorts and Reef Fish in the Maldives. Scripps Institution of Oceanography University of California,
Cowburn B, Moritz C, Grimsditch G, Solandt JL (2019) Evidence of coral bleaching avoidance, resistance and recovery in the Maldives during the 2016 mass-bleaching event. Mar Ecol Prog Ser 626:53–67
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36:561–575
Desbiens AA, Roff G, Robbins WD, Taylor BM, Castro-Sanguino C, Dempsey A, Mumby PJ (2021) Revisiting the paradigm of shark-driven trophic cascades in coral reef ecosystems. Ecology 102:e03303
Dierking J, Williams ID, Walsh WJ (2011) Diet composition and prey selection of the introduced grouper species peacock hind (Cephalopholis argus) in Hawaii. Fish Bull 107:464–476
Dunic JC, Baum JK (2017) Size structuring and allometric scaling relationships in coral reef fishes. J Anim Ecol 86:577–589
Fan Y, Chen J, Shirkey G, John R, Wu SR, Park H, Shao C (2016) Applications of structural equation modeling (SEM) in ecological studies: an updated review. Ecol Process 5:19
FAO (2016) Contributing to food security and nutrition for all. The State of World Fisheries and Aquaculture 2016. FAO (Food and Agriculture Organization of the United Nations). Rome 200
Farmer NA, Ault JS (2011) Grouper and snapper movements and habitat use in Dry Tortugas, Florida. Mar Ecol Prog Ser 433:169–184
Ferrari R, Malcom HA, Byrne M, Friedman A, Williams SB, Schultz A, Jordan AR, Figueira WF (2017) Habitat structural complexity metrics improve predictions of fish abundance and distribution. Ecography 41:1077–1091
Frisch AJ, Ireland M, Baker R (2014) Trophic ecology of large predatory reef fishes: energy pathways, trophic level, and implications for fisheries in a changing climate. Mar Biol 161:61–73
Froese R, Pauly D (2023) FishBase World Wide Web electronic publication. www.fishbase.org, version (03/2023)
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, New York
Grace JB, Anderson MJ, Olff H, Scheiner SM (2010) On the specification of structural equation models for ecological systems. Ecol Monogr 80:67–87
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326
Grimes CB (1979) Diet and feeding ecology of the vermilion snapper, Rhomboplites Aurorubens (Cuvier) From North Carolina and South Carolina Waters. Bull Mar Sci 29:53–61
Heenan A, Hoey AS, Williams GJ, Williams ID (2016) Natural bounds on herbivorous coral reef fishes. Proc Royal Society b: Biol Sci 283:20161716
Hensel E, Allgeier JE, Layman CA (2019) Effects of predator presence and habitat complexity on reef fish communities in The Bahamas. Mar Biol 166:136
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101
Hobson ES (1974) Feeding relationships of Teleostean fishes on coral reefs in Kona. Hawaii Fishery Bulletin 72:915–931
Hobson ES (1979) Interactions Between Piscivorous Fishes and Their Prey. In: Clepper HE (ed) Predator-Prey Systems in Fisheries Management. Sport Fishing Inst, Washington, D.C., pp 231–242
Hu L-t, Bentler PM (1998) Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol Methods 3:424–453
Jankowski MW, Graham NAJ, Jones GP (2015) Depth gradients in diversity, distribution and habitat specialisation in coral reef fishes: implications for the depth-refuge hypothesis. Mar Ecol Prog Ser 540:203–215
Jolliffe IT, Cadima J (2016) Principal component analysis: a review and recent developments. Philosoph Transact Royal Society a: Math, Phys Eng Sci 374:20150202
Karkarey R, Alcoverro T, Kumar S, Arthur R (2017) Coping with catastrophe: foraging plasticity enables a benthic predator to survive in rapidly degrading coral reefs. Anim Behav 131:13–22
Kassambara A, Mundt F (2017) Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.5. https://CRAN.R-project.org/package=factoextra
Kenyon TM, Doropoulos C, Wolfe K, Webb GE, Dove S, Harris D, Mumby PJ (2023) Coral rubble dynamics in the Anthropocene and implications for reef recovery. Limnol Oceanogr 68:110–147
Kramer MJ, Bellwood O, Fulton CJ, Bellwood DR (2015) Refining the invertivore: diversity and specialisation in fish predation on coral reef crustaceans. Mar Biol 162:1779–1786
Kuiter RH (2014) Fishes of the Maldives: Indian Ocean. Atoll Editions, Cairns, Australia
Kulbicki M, Bozec Y-M, Labrosse P, Letourneur Y, Mou-Tham G, Wantiez L (2005) Diet composition of carnivorous fishes from coral reef lagoons of New Caledonia. Aquat Living Resour 18:231–250
Kwan JLY, Chan W (2011) Comparing standardized coefficients in structural equation modeling: a model reparameterization approach. Behav Res Methods 43:730–745
Le S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lefcheck JS, Freckleton R (2016) piecewiseSEM: piecewise structural equation modelling inr for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Lynam CP, Llope M, Möllmann C, Helaouët P, Bayliss-Brown GA, Stenseth NC (2017) Interaction between top-down and bottom-up control in marine food webs. Proc Natl Acad Sci 114:1952–1957
Martínez-Juárez LF, Schmitter-Soto JJ, Cabanillas-Terán N, Mercado-Silva N (2024) Diet variability of snappers (Teleostei: Lutjanidae) in a bay-to-reef ecosystem of the Mexican Caribbean. Water Biol Security 3:100211
Matley JK, Tobin AJ, Simpfendorfer CA, Fisk AT, Heupel MR (2017) Trophic niche and spatio-temporal changes in the feeding ecology of two sympatric species of coral trout (Plectropomus leopardus and P. laevis). Mar Ecol Prog Ser 563:197–210
Matley JK, Maes GE, Devloo-Delva F, Huerlimann R, Chua G, Tobin AJ, Fisk AT, Simpfendorfer CA, Heupel MR (2018) Integrating complementary methods to improve diet analysis in fishery-targeted species. Ecol Evol 8:9503–9515
McCann KS, Rasmussen JB, Umbanhowar J (2005) The dynamics of spatially coupled food webs. Ecol Lett 8:513–523
McCawley JR, Cowan JH Jr, Shipp RL (2006) Feeding periodicity and prey habitat preference of red snapper, lutjanus campechanus (poey, 1860) on Alabama artificial reefs. Gulf Mexico Sci 24(1):4
Mihalitsis M, Bellwood DR (2017) A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE 12:e0184679
Mihalitsis M, Bellwood DR (2019) Morphological and functional diversity of piscivorous fishes on coral reefs. Coral Reefs 38:945–954
Mihalitsis M, Bellwood DR (2021) Functional groups in piscivorous fishes. Ecol Evol 11:12765–12778
Mihalitsis M, Hemingson CR, Goatley CHR, Bellwood DR (2021) The role of fishes as food: A functional perspective on predator–prey interactions. Funct Ecol 35:1109–1119
Mitchell MD, Harborne AR (2020) Non-consumptive effects in fish predator–prey interactions on coral reefs. Coral Reefs 39:867–884
Morais RA, Bellwood DR (2019) Pelagic subsidies underpin fish productivity on a degraded coral reef. Curr Biol 29:1521-1527.e1526
Morais RA, Siqueira AC, Smallhorn-West PF, Bellwood DR (2021) Spatial subsidies drive sweet spots of tropical marine biomass production. PLoS Biol 19:e3001435
Moritz C, Ducarme F, Sweet MJ, Fox MD, Zgliczynski B, Ibrahim N, Basheer A, Furby KA, Caldwell ZR, Pisapia C, Grimsditch G, Abdulla A, Beger M (2017) The “resort effect”: Can tourist islands act as refuges for coral reef species? Divers Distrib 23:1301–1312
Morri C, Montefalcone M, Lasagna R, Gatti G, Rovere A, Parravicini V, Baldelli G, Colantoni P, Bianchi CN (2015) Through bleaching and tsunami: coral reef recovery in the Maldives. Mar Pollut Bull 98:188–200
Neutel AM, Thorne MA (2014) Interaction strengths in balanced carbon cycles and the absence of a relation between ecosystem complexity and stability. Ecol Lett 17:651–661
Oakley-Cogan A, Tebbett SB, Bellwood DR (2020) Habitat zonation on coral reefs: structural complexity, nutritional resources and herbivorous fish distributions. PLoS ONE 15:e0233498
Pisapia C, Burn D, Yoosuf R, Najeeb A, Anderson KD, Pratchett MS (2016) Coral recovery in the central Maldives archipelago since the last major mass-bleaching, in 1998. Sci Rep 6:34720
Pisapia C, Burn D, Pratchett MS (2019) Changes in the population and community structure of corals during recent disturbances (February 2016-October 2017) on Maldivian coral reefs. Sci Rep 9:8402
Podani J, Miklós I (2002) Resemblance coefficients and the horseshoe effect in principal coordinates analysis. Ecology 83:3331–3343
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistial Computing, Vienna, Austria
Rice MM, Ezzat L, Burkepile DE (2019) Corallivory in the anthropocene: interactive effects of anthropogenic stressors and corallivory on coral reefs. Front Marine Sci 11(5):525
Rogers A, Blanchard JL, Mumby PJ (2014) Vulnerability of coral reef fisheries to a loss of structural complexity. Curr Biol 24:1000–1005
Rogers A, Blanchard JL, Newman SP, Dryden CS, Mumby PJ (2018) High refuge availability on coral reefs increases the vulnerability of reef-associated predators to overexploitation. Ecology 99:450–463
Rooney N, McCann K, Gellner G, Moore JC (2006) Structural asymmetry and the stability of diverse food webs. Nature 442:265–269
Rosseel Y (2012) lavaan: an R package for structural equation modeling. J Stat Softw 48:1–36
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67
Russ GR, Aller-Rojas OD, Rizzari JR, Alcala AC (2017) Off-reef planktivorous reef fishes respond positively to decadal-scale no-take marine reserve protection and negatively to benthic habitat change. Mar Ecol 38:e12442
Russ GR, Rizzari JR, Abesamis RA, Alcala AC (2021) Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecol Appl 31:e02224
Sadovy Y (2005) Trouble on the reef: the imperative for managing vulnerable and valuable fisheries. Fish Fish 6:167–185
Safaie A, Silbiger NJ, McClanahan TR, Pawlak G, Barshis DJ, Hench JL, Rogers JS, Williams GJ, Davis KA (2018) High frequency temperature variability reduces the risk of coral bleaching. Nat Commun 9:1671
Sandin SA, Smith JE, DeMartini E, Dinsdale EA, Donner SD, Friedlander AM, Konotchick T, Malay M, Maragos JE, Obura D, Pantos O, Paulay G, Richie M, Rohwer F, Schroeder RE, Walsh SM, Jackson JBC, Knowlton N, Sala E (2008) Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3:e1548
Sandom C, Dalby L, Flojgaard C, Kissling WD, Lenoir J, Sandel B, Trøjelsgaard K, Ejrnæs R, Svenning J-C (2013) Mammal predator and prey species richness are strongly linked at macroscales. Ecology 94:1112–1122
Sasamal SK (2007) Island wake circulation off Maldives during boreal winter, as visualised with MODIS derived chlorophyll-adata and other satellite measurements. Int J Remote Sens 28:891–903
Sattar SA, Andréfouët S, Ahsan M, Adam S, Anderson CR, Lucy C (2012) Status of the Coral Reef Fishery in an Atoll Country Under Tourism Development: The Case of Central Maldives. Atoll Research Bulletin:163–186
Sattar SA, Wood E, Islam F, Najeeb A (2014) Current status of the reef fisheries of Maldives and recommendations for management. . Marine Research Centre/Marine Conservation Society (UK).
Schiettekatte N, Brandl S, Casey J (2019) fishualize: Color Palettes Based on Fish Species R package version 010
Seibold S, Cadotte MW, MacIvor JS, Thorn S, Müller J (2018) The necessity of multitrophic approaches in community ecology. Trends Ecol Evol 33:754–764
Shipley B (2002) Cause and correlation in biology: a user’s guide to path analysis, structural equations and causal inference. Cambridge University Press, Cambridge
Shpigel M, Fishelson L (1989) Food habits and prey selection of three species of groupers from the genus Cephalopholis (Serranidae: Teleostei). Environ Biol Fishes 24:67–73
Siqueira AC, Morais RA, Bellwood DR, Cowman PF (2021) Planktivores as trophic drivers of global coral reef fish diversity patterns. Proc Natl Acad Sci 118:e2019404118
Skinner C, Newman SP, Mill AC, Newton J, Polunin NVC (2019) Prevalence of pelagic dependence among coral reef predators across an atoll seascape. J Anim Ecol 88:1564–1574
Skinner C, Mill AC, Fox MD, Newman SP, Zhu Y, Kuhl A, Polunin NVC (2021) Offshore pelagic subsidies dominate carbon inputs to coral reef predators. Sci Adv 7:eabf3792
Steven P, Newman Erik H, Meesters Charlie S, Dryden Stacey M, Williams Cristina, Sanchez Peter J, Mumby Nicholas VC, Polunin (2015) Reef flattening effects on total richness and species responses in the C aribbean Summary. J Ani Ecol 84(6):1678-1689. https://doi.org/10.1111/1365-2656.12429
St John J (1999) Ontogenetic changes in the diet of the coral reef grouper Plectropomus leopardus (Serranidae): patterns in taxa, size and habitat of prey. Mar Ecol Prog Ser 180:233–246
Stewart BD, Jones GP (2001) Associations between the abundance of piscivorous fishes and their prey on coral reefs: implications for prey fish mortality. Mar Biol 138:383–397
Tickler DM, Letessier TB, Koldewey HJ, Meeuwig JJ (2017) Drivers of abundance and spatial distribution of reef-associated sharks in an isolated atoll reef system. PLoS ONE 12:e0177374
Torres-Pulliza D, Dornelas MA, Pizarro O, Bewley M, Blowes SA, Boutros N, Brambilla V, Chase TJ, Frank G, Friedman A, Hoogenboom MO, Williams S, Zawada KJA, Madin JS (2020) A geometric basis for surface habitat complexity and biodiversity. Nature Ecology & Evolution 4:1495–1501
Williams DM, Hatcher AI (1983) Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Mar Ecol Prog Ser 10:239–250
Williams I, Polunin N (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366
Williams GJ, Smith JE, Conklin EJ, Gove JM, Sala E, Sandin SA (2013) Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1:e81
Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Change Biol 12:2220–2234
Wolfe K, Kenyon TM, Mumby PJ (2021) The biology and ecology of coral rubble and implications for the future of coral reefs. Coral Reefs 40:1769–1806
Wolfe K, Kenyon TM, Desbiens A, de la Motte K, Mumby PJ (2023) Hierarchical drivers of cryptic biodiversity on coral reefs. Ecol Monogr 93:e1586
Yadav S, Fisam A, Dacks R, Madin JS, Mawyer A (2021) Shifting fish consumption preferences can impact coral reef resilience in the Maldives: a case study. Mar PoliCy 134:104773
Acknowledgements
All work was conducted under research permit (OTHR)30-D/INDIV/2016/515 and (OTHR)30-D/INDIV/2018/466 granted by the Republic of Maldives Ministry of Fisheries and Agriculture. Newcastle University Animal Welfare and Ethical Review Body approved the project (project ID: 526). CS was supported by a Newcastle University SAgE DTA studentship and a cooperative agreement with Banyan Tree. We thank Alastair Harborne, Renato Morais, and another anonymous reviewer for their helpful comments that improved the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
CS, NVCP, SPN, and ACM designed the study. CS and SG collected the data. CS, SR, AAD, and ACM analysed the data. CS wrote the first draft of the manuscript, and all authors contributed to revisions.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skinner, C., Gallimore, S., Polunin, N.V.C. et al. Corresponding planktivore and predator spatial distributions in an oceanic coral reef system. Coral Reefs (2024). https://doi.org/10.1007/s00338-024-02514-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00338-024-02514-8