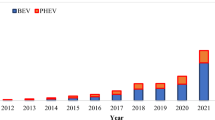
Abstract
The prevalent use of lithium-ion cells in electric vehicles poses challenges as these cells rely on rare metals, their acquisition being environmentally unsafe and complex. The disposal of used batteries, if mishandled, poses a significant threat, potentially leading to ecological disasters. Managing used batteries is imperative, necessitating a viable solution. The remedy lies in implementing robust battery recycling systems. This paper explores diverse disposal methods, particularly focusing on their relevance within the automotive industry, while also acknowledging other potential applications. By adopting a closed-loop approach, this system not only addresses the waste issue but also circumvents environmental costs linked to the extraction and production of new raw materials. Consequently, it not only resolves waste concerns but also mitigates environmental strain and conserves resources. It was described the use of used batteries as energy storage devices. This is an innovative approach to extend battery life cycle, reduce waste and provide cost-effective energy storage solutions. This practice is particularly important for large-scale energy storage systems, such as those used in conjunction with renewable energy sources such as solar and wind energy. The work pays significant attention to battery thermal regimes, which refer to the various temperature conditions in which batteries operate. These systems significantly impact battery performance, lifespan, safety and efficiency. Understanding and managing these thermal regimes is critical to optimizing the use of batteries in a variety of applications, including electric vehicles, portable electronics and renewable energy storage.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We live faster and faster—as our lifestyle changes so does our environment. None of us can imagine functioning without modern technological achievements. However, one should be aware of the threats to the natural environment caused by the development of civilization. From the ecological point of view, the key is to manage waste, recover raw materials, reuse them and safely dispose of them. Given the current technological level of the automotive market, it is necessary to increase the susceptibility of the vehicle to material recovery processes, especially in the case of new types of cars; electric, hybrid, and hydrogen-powered fuel cells [1].
Exploring lithium-ion battery recycling in electric vehicles
The widespread adoption of lithium-ion batteries in electric vehicles (EVs) has propelled advancements in sustainable transportation, offering a promising alternative to traditional combustion engines. However, the proliferation of EVs brings forth a crucial concern: the management of used lithium-ion cells.
As these vehicles age and their batteries degrade, effective recycling becomes imperative.
Lithium-ion cells, containing valuable but finite resources, necessitate careful handling to mitigate environmental impact and optimize resource utilization. This section explores the multifaceted landscape of recycling lithium-ion cells from electric vehicles. It examines the challenges, innovations, and emerging strategies dedicated to responsibly managing and repurposing these batteries, ensuring both environmental sustainability and resource efficiency in the burgeoning era of electric mobility.
Electric cars rely significantly on their batteries, comprised of interconnected cells that store the energy propelling the vehicle. These batteries vary in lifespan, composition, and weight, with lithium-ion batteries currently dominating the market due to their capacity to offer extended ranges, sometimes spanning hundreds of kilometers on a single charge. This technology is not exclusive to the automotive industry, extending its influence across various electronic devices.
However, the escalating demand for these batteries poses a challenge due to the scarcity of lithium and other critical materials. Mining for these resources is costly and environmentally perilous, straining water resources and polluting the environment with waste. Recycling becomes pivotal in balancing this energy ecosystem. Initially, while lithium-ion batteries were produced, effective recycling methods were unknown, significantly inflating manufacturing costs. Today, efforts are directed toward addressing this issue, yet a crucial question lingers: are recycled cells on par with newly mined minerals in performance?
The ramifications are substantial, particularly for electric vehicle manufacturers, who cannot afford diminished battery efficiency or potential damage. Gas emissions during exothermic reactions within these batteries can lead to explosions, emphasizing the necessity for meticulous handling. However, promisingly, recent studies indicate that advanced recycling techniques for cathodes result in batteries performing just as well as those created from scratch, with recycled cathode batteries showcasing extended lifespans and quicker charging capabilities [2,3,4,5,6,7,8,9,10,11]. This underscores the growing viability of recycling in sustaining the performance and longevity of these critical components.
Methods of neutralizing hazardous waste
There are various approaches to neutralizing hazardous waste, aiming to mitigate its environmental impact and potential harm. These methods often involve chemical, physical, or biological processes designed to render hazardous substances less harmful or inert. Common techniques include chemical stabilization, thermal treatment such as incineration, and biological processes such as bioremediation.
Recycling stands as a vital method for neutralizing hazardous waste. This process involves subjecting waste to treatments that facilitate the near-complete recovery of its contained compounds. However, the substantial obstacle to its widespread implementation is the high costs associated with constructing and operating the necessary technological infrastructure. Despite this challenge, as natural resources diminish with the progression of civilization, recycling appears poised to emerge as the primary approach for managing used products in the future.
The imperative to ensure adequate collection and recovery of materials from used or expired cells has spurred the establishment of economic entities specializing in accumulator and battery recycling across several European countries over the past decade.
Depending on the type of waste (cells of one type or a mixture of cells), in the process of recycling batteries, three basic methods of material recovery from used electrochemical power sources are used:
-
mechanical, consisting in shredding the waste in special mills, and then separating the resulting fractions (plastic, paper, etc.),
-
hydrometallurgical, consisting in the recovery of materials as a result of dissolving waste in acids or bases,
-
thermal, consisting in the recovery of materials by smelting metals in special furnaces.
The introduction of an additional step to the above process allows for the recovery of metal oxides (Fe—iron, Mn—manganese, Zn—zinc). The above-mentioned methods of recovering materials from used and expired accumulators and batteries are usually preceded by preliminary stages:
-
collection of used or expired batteries and accumulators,
-
segregation of waste containing various types of accumulators and batteries.
The process of recycling cells (Fig. 1) is legally determined in order not to harm human health and the environment by its improper implementation. Such transformations are applied to each component of the cell, including the temperature-sensitive housing and the electrolyte [12].
Block diagram of the recycling process of lithium-ion batteries [12]
The novelty of the article is primarily to raise the reasons for green chemistry, green materials, which are currently a trend. Extensive research is being conducted on the use of biomaterials as electrodes, e.g., starch, lignin or cellulose. In addition, the topic of cell safety was discussed in the context of describing the exact problems related to EV, and the cell safety system was also thoroughly described, in which attention was paid to how to properly care for the cell. A novelty is certainly the description of the market of recycled batteries.
In addition, a block diagram focusing on the recycling process of lithium-ion batteries was proposed. It is worth paying attention to what substances we are dealing with. It is also important to visualize different batteries for different applications, taking into account their advantages and disadvantages. This is to make people aware of the dangers that may be faced by us in the event of improper disposal.
Proposals, ways of recycling
In response to the pressing need for sustainable solutions in managing the ever-growing stream of used batteries, this section delves into a spectrum of innovative strategies and methodologies for effective battery recycling. As the environmental impact of discarded lithium-ion cells continues to escalate, exploring viable avenues to repurpose and recycle these batteries is paramount. This section aims to elucidate various cutting-edge approaches, from advanced reprocessing techniques to creative applications, fostering a deeper understanding of how we can mitigate environmental harm while harnessing the latent value within these used batteries.
Recycling involves subjecting waste to suitable treatment, enabling the near-complete recovery of its contained compounds. Initially, the cell undergoes a drastic discharge, achieved by applying external resistance or recovering power to the grid, especially in larger-scale processes. Following discharge, battery packs are dismantled into individual cell components, safely fragmented via an inert gas flow. At this stage, the partially vaporized electrolyte is collected through condensation.
The result is that the major proportion remaining in the particulate material is recovered by the three considered methods:
-
Thermal drying, no recovery of salt, which is the highest value of the electrolyte;
-
Flow using subcritical carbon dioxide assisted by electrolyte extraction with a co-solvent (acetonitrile) to elute conductive salt;
-
Static supercritical carbon dioxide assisted with electrolyte extraction without co-solvent [13].
Magnetic separation removes metallic iron-containing elements [e.g., LiFePO4—lithium iron(II) phosphate]. The remaining non-magnetic materials undergo separation in a zigzag air classifier or a cross-flow separator. Air is used to segregate a fine fraction containing mostly electrodes and a heavier fraction holding separators and plastics.
The second part then enters a furnace heated to 400–600 °C to break down binders (mainly PVdF—polyvinylidene fluoride). This process significantly aids in detaching active material particles from their current collectors. Subsequently, multiple rounds of air-jet screening detach and separate the active materials from each other.
The metallic fraction, comprising aluminum and copper collectors, undergoes separation based on differing material densities. Specialized recycling plants handle this separation process to effectively remove these materials.
At this stage, the graphite is removed from the recycling process. The cathode fraction, containing mainly LiNi1/3Co1/3Mn1/3O2 (along with iron, copper and aluminum) is subjected to hydrometallurgical separation and refining processes. During this step, lithium is leached from the cathode material by dissolving it in sulfuric (VI) acid containing hydrogen peroxide. While materials containing nickel, cobalt and manganese are dissolved, the dipositive iron ions from LiFePO4 are oxidized by hydrogen peroxide to Fe3+. In this process an insoluble FePO4 salt and sediment are formed. Then the copper and aluminum are precipitated by appropriate adjustment of the pH (4–6). After the filtration process, the pH is adjusted again (below 9) and the transition metals are precipitated as hydroxides, while the lithium remaining in the solution can be converted into a concentrated LiOH—lithium hydroxide monohydrate—solution using bipolar membrane electrodialysis (EDBM), which after drying can be used for cathode synthesis. The demanganization process is carried out by oxidation. To achieve this, the atomized air is introduced into the Mn2+ solution, and then the MnO2 (manganese (IV) oxide) is precipitated and filtered. Ni2+ and Co2+ cannot be separated by precipitation, so they must be extracted using complex agents such as di(2,4,4-trimethylpentyl)-phosphinic acid (so-called CYANEX 272). In two consecutive mixer-settlers, the solutions can be separated into NiSO4 (nickel(II) sulfate hexahydrate) and CoSO4 (cobalt(II) sulfate). After drying, the obtained precursors LiOH, Mn(OH)2, Ni(OH)2, Co(OH)2 are mixed together in a stoichiometric ratio (usually 3:1:1:1). Then, they are introduced into the furnace to re-synthesize the LiNi1/3Co1/3Mn1/3O2 cathode material [13].
Various alternative methods for recycling active materials have been explored in literature, such as the leaching technique using different acids: oxalate [14], ascorbic [15], or citric [16], as well as concentrated acids [17,18,19].
However, it is crucial to note that the ideal recycling process should solely involve separation and delamination. In reality, this procedure is considerably more intricate. A significant challenge lies in effectively opening the battery pack while maintaining its mechanical integrity. This method should not only facilitate easy access but also ensure durability [24,25,26]. Some researchers have delved into this area and proposed automated solutions. Nonetheless, a major hurdle remains the lack of standardization in battery packaging; different manufacturers employ various pack configurations [20,21,22,23].
Battery usage overview—market of electric vehicles
Lithium-ion cells are widely used in Electric Vehicles due to their lightweight nature, extended range, shape adaptability, and the ability to be charged even when not fully depleted. However, their main drawbacks are the higher cost and potential flammability. Figure 2 outlines various rechargeable and primary battery types along with their respective advantages, disadvantages, and applications.
Different batteries for different applications with pros and cons [27]
In EVs, hybrid systems such as lithium-ion capacitors [27] can be assembled, but presently, lead acid, Ni–MH, and Ni–Cad batteries remain more prevalent. It's worth noting that Ni–MH and Ni–Cad batteries [28] are susceptible to memory effects and are less environmentally friendly compared to lithium-ion batteries [27,28,29,30].
It should be noticed that nowadays, there are new electric vehicle’s standards [27] and thus recycled EV-battery standards which are summarized in Fig. 3. Total market value for batteries and recycled batteries is equal to 15,000 Euro/t, 1000–3000 Euro/t, respectively. The most valuable materials are copper, lithium, cobalt and nickel, which pose 30% of the whole battery.
As it could be seen in Fig. 4, the recycling process consists of four main stages: materials collection and preparation, material refining, battery manufacturing and EV assembly and sale. The main players are recyclers, giga factories, automakers, chemists, mining and refining, metallurgists, energy providers. The main actors in Europe are SNAM, Fortum, Duesenfeld, Neometals, Suez-Eramet-BASF, Veolia-Solvay, Umicore, Northvolt (the biggest one).
Recycling competitors in Europe [27]
In EVs the most widely used battery is NMC 111 type (LiNi1/3Mn1/3Co1/3O2). The battery system perimeter (volume) makes up the majority of this cell.
The production of new Electric Vehicles is anticipated to witness a substantial surge in demand. In 2020, Europe experienced a remarkable year for EVs. The UK's plugin electric vehicle market notably soared, achieving a market share of 23.4% in December 2020, a significant increase from 6.6% in December 2019. Notably, the Tesla Model 3 emerged as the top-selling vehicle across all categories in December.
Sweden also reached a milestone, hitting a record 50% electric vehicle share in December. Meanwhile, Norway continues to set exceptional records, with a consistent market share for electric vehicles; in December, plugin electric vehicles achieved an astonishing 87.1% market share. France, Germany, and several other markets also saw impressive sales figures.
Veolia stands out as a key partner in this domain. Their comprehensive capabilities encompass utilities plant management, water cycle services, site-wide mechanical and electrical services, energy management, and comprehensive waste management encompassing both hazardous and non-hazardous waste. Their services include optimal operation and maintenance strategies, as well as robust design and build capabilities, with potential financial backing [30].
Figure 5 illustrates the sequential steps involved in recycling electric vehicle batteries on a larger scale. This process encompasses multiple stages: battery first and second life, collection, dismantling, physical and chemical materials separation, materials purification, precursor production, and the assembly of new batteries.
Exploring both environmental and economic dimensions of batteries
To mitigate environmental impact and enhance environmental protection efforts, several challenges need addressing: efficiency, productivity, sustainability, compliance, safety, and financial engineering aspects (refer to Fig. 6). Factories are proposing four key actions to tackle these issues: maximizing waste utilization [28], adopting greener energy sources [29], minimizing water usage, and collaborating to complete the battery life cycle loop [30].
Partner to reduce gigafactory’s environmental footprint and close the loop of battery life cycle [28]
It's essential to consider the environmental impact of recycling. The recycling process consumes energy and releases pollutants, primarily CO2, into the atmosphere. The emission levels vary based on the recovery rate, determined by the recycling technology employed [28].
In the case of the hydrometallurgical recycling method, substantial amounts of acids are required during the leaching process. Proper management of the resulting sludge is necessary for reuse or proper disposal. However, it's crucial to note that extracting metals from natural deposits also entails emissions and costs linked to energy supply for mining machinery and equipment [30].
To evaluate the economic and environmental implications, a comparison was conducted among three types of lithium-ion batteries, assessing whether extraction or recycling is more favorable (see Table 1):
Table 1.
The recycling technologies employed are advanced, allowing for the environmentally safe processing of waste. According to data [31], it is evident that, at comparable costs, the production of new cells can occur without disrupting the natural environment. Furthermore, recycling electric vehicle batteries emerges as a highly ecological solution. The sources of rare earth metals are non-renewable, and their extraction poses both difficulty and environmental harm. Inevitably, the electric vehicle industry will need to transition towards using materials obtained through recycling. Consequently, recycling methods will continue to evolve and expand, resulting in cost-effective and more environmentally friendly solutions [31].
Challenges of recovery of valuable battery parts for used cells and their possible application
The second phase of the project is to create a mechanism for the efficient recycling of batteries used to build energy storage systems by recovering and reusing valuable metals (Fig. 7).
Waste batteries as energy storage systems—Toyota and CHUBU Electric Power were the first to start such a project. The project consists of two phases, the first is the creation of the Storage Battery System, consisting of waste batteries from electric and hybrid vehicles produced by Toyota. Work will begin this year 2023, and by 2030 the system is to achieve electricity production capacity of 10 thousand tonnes kilowatts. The introductory phase will include research related to the recycling of the most popular NI–Mh batteries. Around 2030, the scope of work is also to be extended to lithium-ion batteries.
One of the major challenges associated with recycling lithium-ion batteries is waste management; however, it is inaccurate to claim that all batteries currently produced end up in landfills. When a lithium-ion battery reaches the end of its operational life, it still retains around 80% of its charge. While this might not be sufficient to power an electric vehicle, it proves suitable for various other applications, such as energy storage. These secondary batteries can be utilized for at least 10 years. The process of reuse is generally more cost-effective than recycling and is also more environmentally friendly [32, 33].
As these batteries often contain precious materials such as nickel, manganese, and cobalt, disposing of them in landfills as waste would be a squandering of valuable resources, especially considering the increasing volume of waste. "If the purpose of owning electric cars is to reduce carbon dioxide emissions, then extracting these raw materials from the ground to produce electric vehicles contradicts the very reason for owning them." A robust recycling infrastructure could also mitigate the depletion of critical cobalt reserves necessary for battery production; it is estimated that recycling could contribute 10% of Europe's cobalt supply by 2030 [34].
The recycling process, for the most part, involves a combination of pyrometallurgy and hydrometallurgy, but this method has its limitations. While it successfully recovers some of the battery's most valuable metals, many other precious materials are lost. The typical approach involves placing the battery into a smelter, resulting in a mix of alloys at the bottom—usually nickel, cobalt, and copper. After lithium and aluminum end up in landfills, metal alloys remain, requiring hydrometallurgical treatment to extract maximum value. This involves breaking the cathode crystal structure and leaching various ions from the battery to obtain precursor salts like nickel sulfate and cobalt sulfate, which can be used to manufacture new batteries. "Cobalt is by far the most valuable product." Despite automobile manufacturers transitioning to battery chemistry with lower cobalt content, the value obtained from the leaching process is continually diminishing [35].
There are a number of problems that complicate the layout of extra efficient manner. The underlying trouble is that those batteries are clearly now not recyclable, they're designed for high overall performance and sturdiness—basic battery homes that may be compromised at the price of designing a battery that is extra recyclable. A lithium-ion battery percent includes several thousand cells grouped into modules, with every cell containing a cathode, anode, separator and electrolyte. Cathodes commonly consist of a lively transition metallic oxide powder blended with carbon black and adhered to an aluminum foil modern collector with a compound along with polyvinylidene fluoride. The anodes incorporate graphite adhered to the copper foil by PVdF, and the electrolyte is mostly a LiPF6 salt answer. Its miles this type of massive wide variety of factors, in addition to the manner they're assembled, that makes setting apart them tough [36].
Because of this, the most inexpensive and common method of recycling batteries is their destruction. However, there is no standard battery for electric cars; popular chemical compositions include nickel–cobalt–aluminum, lithium–manganese oxide, nickel–magnesium–cobalt oxide, among others. Each car model features a different battery structure. When destroying a battery, these diverse compounds must be separated. This can be achieved through density separation or by exploiting the distinct magnetic properties of various metal oxides.
The assembly process means that when shredding, one of the most valuable components—the cathode "black mass," or the active electrochemical metallic oxide—comes out contaminated with other materials. To address this issue, scientists are exploring ways to design packages, modules, and cells so that they are easier to dismantle at the end of their life cycle, eliminating the need for shredding or simplifying the cleaning process. This might involve simple changes like replacing welds with nuts and bolts or working on the development of new adhesives that are easier to handle.
Even in the absence of a recycling assignment, scientists are exploring approaches to maximize recovery. A hydrometallurgical method would be more efficient—utilizing aqueous solutions to leach valuable metals from the cathode material after grinding. Typically, this is achieved using a combination of sulfuric acid and hydrogen peroxide, with the peroxide acting as a reducing agent to transform insoluble cobalt. After leaching, cobalt and lithium can be recovered as a salt by precipitating the solution through a change in pH. This process yields a high-purity starting material that can be used in the production of new batteries. However, while the hydrometallurgical process recovers more valuable substances than those used solely in the pyrometallurgical process, the cost of the cathode material is not fully recovered. Therefore, while it is beneficial for cathodes containing high amounts of cobalt, such as lithium cobalt oxide—where hydrometallurgical processes can recover up to 70% of the value of these cathodes—it is less advantageous for cathodes that are less rich in cobalt. In such cases, where most of the values lie in the cathode oxides themselves and not in the raw materials [37, 38], hydrometallurgical processes may be less practical.
Safety of lithium-ion cells
Introduction
Lithium-ion batteries are a relatively new technology. However, since their introduction in the early 1990s, they have had a lasting impact on the energy storage market and are gradually replacing old technologies. Today, it is impossible to imagine our daily life without lithium batteries—and for good reason: they are particularly small and at the same time very efficient and therefore interesting for a wide range of applications. Not only do smartphones and tablets get their energy from lithium batteries, they also play an important role in the field of electromobility. Lithium energy storage systems score points here thanks to, among other things, high energy density with low curb weight and fast charging [39].
Over the past few years, issues related to lithium-ion batteries have become increasingly common. Although there is no single recognized cause of ignition in lithium-ion batteries, these incidents share common features. One such factor is the use of unapproved or aftermarket batteries. It's convenient to purchase spare batteries from third parties online. However, these batteries might lack the necessary third-party certification, and some may even be counterfeit. Such batteries may not undergo proper testing and verification to ensure they are manufactured correctly and meet all relevant safety standards and regulations. Even if a battery adheres to safety standards, it may not be certified to conform to the specific device, making it potentially hazardous.
Another characteristic of lithium-ion battery explosion incidents is the manufacturing process. Most electronic device manufacturers source lithium-ion batteries from suppliers. It's crucial for the device manufacturer to ensure that the battery supplier has implemented and maintains appropriate quality systems. This includes confirming whether the battery is constructed correctly, is free from contamination, and is stored and transported at suitable temperatures. Rigorous monitoring must occur throughout the entire supply chain to ensure that cells and other components comply with the relevant safety standards (see Fig. 8).
Battery thermal regimes
Nevertheless, special care should be exercised when handling lithium-ion batteries, as incidents of dangerous fires can occur. When such events occur, the consequences are often devastating. The peril emanates from the battery's design itself. When high-energy–density materials and highly flammable electrolytes come into contact, they create a mixture that poses a significant fire hazard. The situation becomes particularly hazardous when a lithium battery releases its stored energy in an uncontrolled manner. Once the heat generated surpasses the melting point of the separator, an uncontrollable chain reaction ensues, leading to a catastrophic "thermal runaway."
The high thermal energy initially causes the electrolytic fluid to evaporate, generating additional heat and flammable gases. If the pressure surpasses a certain threshold, flammable gases are released, forming a combustible mixture with air—resulting in flames on the exterior of the battery. The heat exhaustion of a single cell is sufficient to raise the adjacent cells' temperature in a battery block to the point where a violent chain reaction occurs. Once this process begins, it only takes a few minutes for the battery to undergo explosive combustion. Controlling these lithium-ion battery fires is challenging, and the fire tends to spread rapidly. Often, the fire department's role is limited to protecting nearby areas [40].
Due to its highly reactive nature, metallic Lithium (Li) poses a risk of fire and explosion. To mitigate dendritic desegregation (a process causing dangerous damage to the cell), the metallic lithium in the anode is replaced with lithium intercalate. This results in lithium forming compounds with oxygen, such as cobalt or manganese. Plastics and polymers are then utilized to stabilize the electrolyte and the housing structure. In emergency conditions, the release of flammable gases (e.g., C2H4, C2H6) and the occurrence of a fire are possible. In addition, metallic lithium (used in disposable batteries) releases hydrogen when in contact with water, the chemical compounds in the cells' construction contain substantial amounts of oxygen, lithium dust poses a specific risk of explosion and fire. After extinguishing, there is a risk of re-ignition. Discussing the fire hazards associated with lithium battery technology in detail is challenging due to changes in design solutions and recycling methods, which undoubtedly contribute to cost reduction.
Figure 9a illustrates the battery protection system, comprising an internal system (separators, anode, cathode, electrolyte, closing ceramics), external components (referred to as modular safety and BMS), and passive protection. The BMS (battery management system) serves as an electronic safeguard against overcharging or discharging of the cell. It also plays a crucial role in managing overcurrents during both charging and battery operation. These systems typically involve integrated circuits that utilize specialized materials in their construction. In elevated temperatures, the cells undergo expansion, filling up with gas resulting from the decomposition of ethyl and dimethyl carbonates, namely, carbon monoxide (IV). Phones and laptops employ sophisticated SMT (Surface Mount Technology) systems, which oversee operational parameters, including the number of cycles, capacity, and temperature [41].
Battery safety system (a); (b) factors influencing heat release in LIBs [41]
When discussing the LIB battery protection system, it should be noted how the cell should be properly discharged. Deep discharge leads to a copper plating reaction, then causing an internal short circuit and overheating, which may result in the appearance of smoke, sparks. A typical single cell voltage is around 3.6 V, which starts to drop during operation. When the potential is reduced to 2.4–2.5 V, the cell may be irreversibly damaged. In portable devices, there are signals of full battery discharge. When the link is too long in a discharged state, the device may not restart. Do not discharge the battery with too much current. Lithium-ion cells should be recharged when they reach about 60% of their output capacity, because there is no so-called memory effect. It is associated with a sudden loss of capacity. When not in use for a long time, lithium-ion batteries should be stored correctly at low temperatures. They should then have half the output capacity. A current of 0.2–0.3 of the nominal cell capacity should be used. For example, when the charging capacity of a cell is 2000 mAh, a current in the range of 400–600 mA should be used and charged with a power supply to a voltage of 4.2 V.
The current should gradually decrease to a value of 0 mA. The cell undergoes charging through the CC–CV process, denoting constant current–constant voltage. Initially, the cell is charged to a specific maximum level using a constant current (CC). Subsequently, the device undergoes recharging at constant voltage (CV) and alternating current. The heat generated during the exothermic reactions (Fig. 9b) significantly accelerates the reactions occurring inside the cell, leading to a continuous rise in its temperature. In addition, controlling the temperature change over time is challenging, and an increase in temperature within a single cell may impact the enthalpy of serially connected cells. Improper use of batteries can result in electrolyte leakage, gas emission, smoke, sparks, debris, flames, deflagration, and explosions.
Towards green technology
Electric vehicles (EVs) are now being implemented on industrial scale because of green chemistry aspects and environmental protection. There are still some issues when it comes to the infrastructure, production, recycling and green rules. It should be noted, that we can distinguish 12 green chemistry rules that are highly important [24,25,26]:
-
Prevent waste;
-
Atom economy;
-
Less hazardous chemical synthesis routes;
-
Designing of safer chemicals;
-
Designing of safer solvents and auxiliaries;
-
Design for energy efficient;
-
Use of renewable feedstocks;
-
Reduce derivatives;
-
Catalysts–catalytic reagents (as selectively as possible) are superior to stoichiometric reagents.
-
Design for degradation;
-
Real-time analysis for pollution prevention;
-
Inherently safer chemistry for accident prevention.
Figure 10 shows the development and manufacturing goals of lithium-ion cells, including cost, performance, and sustainability aspects. At present, the key performance factors (left) outweigh the key aspects of sustainability (right). Batteries are characterized mainly by the price and several key performance indicators (KPIs), incl. specific energy (Wh kg−1), energy density (Wh L−1), specific power (W kg−1), power density (W L−1). These are also cyclic and calendar life, safety, and charging time (ability to quickly charge). Taking these aspects into account, the main focus in recent decades has been on efficiency improvements and cost reduction [42]. It was possible mainly because of the use of cheaper elements, chemicals and recovery systems of some materials. The sustainable development led to reduction of carbon print and enabled use of renewable sources.
Schematic illustration of the development and production goals of current LIBs, based on [42]
In recent years, an attempt has been made to eliminate cathode (aluminum) and anode (copper) current collectors and expensive binders used in LIBs. One solution is the application of inexpensive 2 mm long natural cellulose fibers as binder and substrate for the electrode. The purpose of this innovation is to eliminate the use of heavy and inactive current-collecting films as collectors and to replace conventional expensive binders with cellulosic fibers. Moreover, no harmful solvents are used in the production of the film. Water-soluble carbons are also used to reduce the preparation time and to obtain a better uniform distribution of the carbon in the electrode. This improves electrochemical performance. Flexible and resistant LiFePO4 (LFP), Li4Ti5O12 (LTO), organic 3,4,9,10-perylene tetracarboxylic dianhydride (PTCDA), and graphite electrodes are obtained with similar active mass charges to those obtained by casting. An initial discharge capacity of approximately 130 mAh g−1 at 2 °C is obtained for a paper LiFePO4/Li4Ti5O12 battery with approximately 91.6% capacity after 1000 cycles. In addition, the fully organic prelithiated PTCDA/graphite cell without transition metal is subjected to electrochemical testing. It is one of the first self-contained batteries to consist of organic redox-active molecules and biodegradable ingredients [43].
Another problem is the commonly used PVDF binder and the paste-like filler.
[N-methyl-2-pyrrolidone (NMP)] used therein. It should be noted that this filler is expensive and is also a very toxic solvent, which contradicts the use of green chemistry slogans. NMP is also involved in recycling. Thus, its function is extremely important and therefore difficult to replace with other materials. Scientists tried to replace this solvent with an aqueous solution, while PVdF—a dispersion of a latex binder in the cathode and a water-soluble styrene–butadiene in the anode. It brought action similar to classic cells, but it should be remembered that the use of water is very dangerous due to the reactive lithium and hydrogen from cathode ingredients evolution [44]. Some research data show the use of water-based binders for cathode systems. In work [45] sodium alginate (SA) and mixed PVDF/carboxymethyl cellulose (CMC) for LTO cathode was applied. In the study [46] SA and CMC/PTFE for LiFePO4/C (LFP/C) cathode were examined. The authors showed that the main reason for positive results is homogenous coverage of acetylene black (AB) on LFP cathode, which offers a great conductive network.
Another solution that increases the safety of battery use is the elimination of toxic organic solvents. They are unfortunately commonly used in commercial cells. Another disadvantage is their high flammability, which reduces the overall permissible operating temperature, followed by thermal runaway. Such solutions are composite polymer–ceramic electrolytes, polymeric ionic liquids, and ionic liquids (ILs). These are also polymer electrolytes. Here gel polymer electrolytes (GPEs), solvent-free polymer electrolytes (SPEs), and composite polymer electrolytes (CPEs) may be highlighted [47,48,49,50,51]. It has to be underlined that still, a big problem remains too low ionic conductivity. Finally, current solutions attempt to improve biodegradability and use wastes, to make the batteries safer. However, the more green battery is the more expensive the preparation of materials becomes on a higher scale.
Recycling lithium-ion batteries involves several processes aimed at recovering valuable materials like cobalt, nickel, and lithium while ensuring minimal environmental harm. Innovations in recycling techniques, such as hydrometallurgical and pyrometallurgical methods, have shown promise in efficiently extracting and purifying these materials for reuse in new batteries.
Safety aspects are crucial throughout the lifecycle of lithium-ion batteries. During recycling, it is important to manage potential risks, like thermal runaway or hazardous chemical exposure, which can arise from damaged or degraded batteries. Implementing safe dismantling procedures, proper storage, and effective handling of electrolytes and cathode materials are essential to minimize these risks.
In addition, developing standardized safety protocols and regulations specifically tailored to the recycling of lithium-ion batteries is crucial. This ensures the protection of workers, the environment, and the overall sustainability of the battery recycling process.
Exploring these novel recycling technologies alongside stringent safety measures not only addresses the burgeoning issue of e-waste but also fosters a more sustainable approach to harnessing the potential of lithium-ion batteries in the electric vehicle industry.
Conclusions
Increasing consumerism and technological advancements are contributing to a surge in the production of cars, making the automotive industry the fastest-growing sector.
The challenge lies in the limitations of existing recycling methods, with companies unprepared to handle the increasing volume of batteries for recycling.
Addressing the environmental footprint involves overcoming industrialization issues related to safety and efficiency. Using greener energy, such as water instead of flammable liquids, natural polymer-based materials for binders and separators, and adopting solid electrolytes or ionic liquids, can help tackle these challenges.
The work presents several proposals on how to address these issues and design an effective recycling process. Furthermore, it is worthwhile to introduce additional changes.
The crucial issue is the packaging of cells for easy dismantling, and current solutions are still not optimal. Ensuring a special atmosphere and safety aspects during cell opening is essential. The common method for battery disassembly involves destruction and density separation to obtain metallic compounds.
Safety concerns with lithium-ion batteries, particularly the flammable electrolyte–solvent mixture, raise doubts about the green energy aspects. Contaminations, electrode defects, moisture, adhesion, storage and transportation temperatures, and the potential for short circuits are critical factors. Deep battery discharge leads to a current collector plating reaction, resulting in short circuits and sparks.
Pure lithium is not used in batteries due to safety concerns; instead, lithium intercalate with lower energy density is employed.
Utilizing green materials like cellulose derivatives, water, and new electrolyte classes such as solid electrolytes or ionic liquids can reduce material costs. However, green electrolytes face challenges in terms of lower conductivity and a fundamentally different industrial production method, mainly through calendaring and extrusion.
In summary, recycling lithium-ion batteries from electric vehicles in industrial production faces challenges in terms of costs, efficiency, and environmental impact. The future focus on safety involves exploring solid electrolytes, which are currently in the startup phase and still encounter industrialization issues.
References
Pigłowska M, Kurc B, Galiński M, Fuć P, Kamińska M, Szymlet N, Daszkiewicz P (2021) Challenges for safe electrolytes applied in lithium-Ion cells—a review. Mater 14(22):6783
Weyhe, R.; Desmuee, I.; Tedjar, F. 2012 Economic Requirements on Future Li-ion Recycling Processes. Workshop: Insights into Novel Solid Materials, their Recyclability and Integration into Li Polymer Batteries for EVs—Future Research in this Field. Timisoara
Friedrich, B.; Vest, M.; Wang, H.; Weyhe, R. 2011. Processing of Li-based Electric Vehicle Batteries for Maximized Recycling Efficiency. In: 16th International Congress of Battery Recycling ICBR, Venice
Li J, Shi P, Wang Z, Chen Y, Chang CC (2009) A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 77(8):1132–1136
Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutz M (2012) Development of a recycling process for Li-ion batteries. J Power Sour 207:173–182
Granata G, Moscardini E, Pagnanelli F, Trabucco F, Toro L (2012) Product recovery from Li-ion battery wastes coming from an industrial pre-treatment plant: lab scale tests and process simulations. J Power Sour 206:393–401
Chen L, Tang X, Zhang Y, Li L, Zeng Z, Zhang Y (2011) Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108(1–2):80–86
Li L, Zhang X, Li M, Chen R, Wu F, Amine K, Lu J (2018) The recycling of spent lithium-ion batteries: a review of current processes and technologies. Electrochem Energy Rev 1:461–482
Heelan J, Gratz E, Zheng Z, Wang Q, Chen M, Apelian D, Wang Y (2016) Current and prospective Li-Ion battery recycling and recovery processes. Jom 68(10):2632–2638
Zeng X, Li J, Liu L (2015) Solving spent lithium-ion battery problems in China: opportunities and challenges. Renew Sustain Energy Rev 52:1759–1767
Bernardes AM, Espinosa DCR, Tenório JAS (2004) Recycling of batteries: a review of current processes and technologies. J Power Sour 130:291–298
Zhang X, Xie Y, Lin X, Li H, Cao H (2013) An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J Mater Cycles Waste Manag 15:420–430
Rothermel S, Evertz M, Kasnatscheew J, Qi X, Grützke M, Winter M, Nowak S (2016) Graphite recycling from spent lithium-ion batteries. Chemsuschem 9(24):3473–3484
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 32:1575–1582
Li L, Lu J, Ren Y, Zhang XX, Chen RJ, Wu F, Amine K (2012) Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sour 218:21–27
Li L, Ge J, Wu F, Chen R, Chen S, Wu B (2010) Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J hazardous mat 176:288–293
Ferreira DA, Prados LMZ, Majuste D, Mansur MB (2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J Power Sour 187:238–246
Shin SM, Kim NH, Sohn JS, Yang DH, Kim YH (2005) Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79(3–4):172–181
Dorella G, Mansur MB (2007) A study of the separation of cobalt from spent Li-ion battery residues. J Power Sources 170:210–215
Waldmann T, Iturrondobeitia A, Kasper M, Ghanbari N, Aguesse F, Bekaert E, Wohlfahrt-Mehrens M (2016) Post-mortem analysis of aged lithium-ion batteries: disassembly methodology and physico-chemical analysis techniques. J Electrochem Soc. https://doi.org/10.1149/2.1211609jes
Herrmann C, Raatz A, Mennenga M, Schmitt J, Andrew S (2012) Assessment of automation potentials for the disassembly of automotive lithium ion battery systems. Springer, Leveraging technology for a sustainable world, pp 149–154
Herrmann C, Raatz A, Andrew S, Schmitt J (2014) Scenario-based development of disassembly systems for automotive lithium ion battery systems. Adv Mater Res 907:391–401
Das A, Li D, Williams D, Greenwood D (2018) Joining technologies for automotive battery systems manufacturing. World Elec Veh J. https://doi.org/10.3390/wevj9020022
Notter DA, Gauch M, Widmer R, Wager P, Stamp A, Zah R, Althaus HJ (2010) Contribution of Li-Ion batteries to the environmental impact of electric vehicles. Environ Sci Technol 44(17):6550–6556
Thompson DL, Hartley JM, Lambert SM, Shiref M, Harper GD, Kendrick E, Abbott AP (2020) The importance of design in lithium ion battery recycling–a critical review. Green Chem 22(22):7585–7603
Wang R, Feng L, Yang W, Zhang Y, Zhang Y, Bai W, Guan H (2017) Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res Lett 12(1):1–11
Williamson, S.S.; Cassani, P.A.; Lukic, S.; Blunier, B. 2011 Energy Storage. Power Electronics Handbook. 1331–1356.
Li J, Wang G, Xu Z (2016) Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries. Waste Manag 52:221–227
Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutzc M (2012) Development of a recycling process for Li-ion batteries. J Power Sources 207:173–182
Rimpas D, Kaminaris SD, Aldarraji I et al (2021) Energy management and storage systems on electric vehicles: a comprehensive review. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2021.08.352
Qi Z, Koenig GM (2017) Review article: flow battery systems with solid electroactive materials. J Vac Sci Technol 35:040801
Motapon SN, Lachance E, Dessaint L-A, Al-Haddad K (2020) A generic cycle life model for lithium-ion batteries based on fatigue theory and equivalent cycle counting. IEEE Open J Ind Electron Soc 1:207–217
Zhang D, Dey S, Perez HE, Moura SJ (2020) Real-time capacity estimation of lithium-ion batteries utilizing thermal dynamics. IEEE Trans Control Syst Technol 28:992–1000
www.laboratoryjnie.pl ISSN 2719–7859-Cordis.europa.eu New cobalt recycling methods pave the way to a greener future
Barré A, Deguilhem B, Grolleau S, Gérard M, Suard F, Riu D (2013) A review on lithium-ion battery ageing mechanisms andestimations for automotive applications. J Power Sour 241:680–689
Zhang P, Liang J, Zhang F (2017) An overview of different approaches for battery lifetime prediction. IOP Conf Ser Mater Sci Eng 199:012134
Sedlakova V, Sikula J, Majzner J, Sedlak P, Kuparowitz T, Buergler B, Vasina P (2016) Supercapacitor degradation assesment by power cycling and calendar life tests. Metrol Meas Syst 23:345–358
Mendis N, Muttaqi KM, Perera S (2014) Management of low-and high-frequency power components in demand-generationfluctuations of a DFIG-based wind-dominated RAPS system using hybrid energy storage. IEEE Trans Ind Appl 50:2258–2268
Guan W, Huang X (2021) A Modular active balancing circuit for redox flow battery applied in energy storage system. IEEE Access 9:127548–127558
Yuan, X.; Liu, H.; Zhang, J. 2011 Ion Batteries, Adv. Mat. and Technol. CRC Press. 198–206.
Korthauer R (2018) Lithium-Ion Batteries Basics and Applications. Springer, pp 103–292
Dühnen S, Betz J, Kolek M, Schmuch R, Winter M, Placke T (2020) Toward green battery cells: perspective on materials and technologies. Small Methods. https://doi.org/10.1002/smtd.202000039
Delaporte N, Lajoie G, Collin-Martin S, Zaghib K (2020) Toward low-cost all-organic and biodegradable Li-ion batteries. Sci Rep 10:3812–3820
Li J, Lu Y, Yang T, Ge D et al (2020) Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes—a green and sustainable manufacturing system. iScience 23(5):101081–101084
Toigo Ch, Arbizzani C, Pettiner K-H, Biso M (2020) Study on different water-based binders for Li4Ti5O12 electrodes. Molecules 25:2443–2445
Zhang X, Ge X, Shen Z, Ma H, Wang J et al (2021) Green water-based binders for LiFePO4/C cathodes in Li-ion batteries: a comparative study. New J Chem 45:9846–9855
Arya A, Sharma AL (2017) Polymer electrolytes for lithium-ion batteries: a critical study. Ionics 23:497–540
Cheng E.J., T. Kimura, M. Shoji, et al., 2020 Ceramic-Based Flexible Sheet Electrolyte for Li Batteries 12 10382-10388.
Boaretto N, Meabe L, Martinez-Ibañez M et al (2020) Review—polymer electrolytes for rechargeable batteries: from nanocomposite to nanohybrid. J Electrochem Soc 167:70524
Yue L, Ma J, Zhang J et al (2016) All-solid-state polymer electrolytes for high-performance lithium-ion batteries. Energy Storage Mater 5:139–164
Rajendran S, Sivakumar M, Subadevi R, Nirmala M (2004) Characterization of PVA–PVdF based solid polymer blend electrolytes. Phys B Condens Matter 348:73–78
Acknowledgements
This work was supported by the Polish Ministry of Science and Higher Education, grant 0911/SBAD/2302.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pigłowska, M., Kurc, B., Fuć, P. et al. Novel recycling technologies and safety aspects of lithium ion batteries for electric vehicles. J Mater Cycles Waste Manag (2024). https://doi.org/10.1007/s10163-024-02028-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10163-024-02028-z