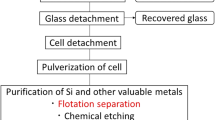
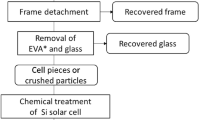
Abstract
In this study, floatability rate of aluminum (Al) powders was analyzed for the purpose of separating valuable resources from residual materials in waste photovoltaic (PV) solar cells, and equations for flotation recovery were developed for various flotation types according to the rate-determining steps of the gas flowrate and feed rate. The flotation rate became a zero-order reaction at the rate-determining step of the gas flow rate and had the same form between a batch and continuous typed practices by substituting residence time with real time. Under the rate-determining step of the feed rate, the flotation rate was expressed by the linear combination of the first-order reaction of an even group material. The flotation recovery rate of Al powders was analyzed by the data of a batch floatability experiment and indicated by the linear expression of the first-order reaction of two groups due to the rate-determining step of the feed rate. The calculated separation recovery of n-cell type device increased as the number of cells increased and approached that of the batch and column types.








Similar content being viewed by others
References
Union E (2012) Directive 2012/19/EU of the European parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment (WEEE). Off J Eur Union 197:38–71
Dias P, Schmidt L, Gomes LB, Bettanin A, Veit H, Bernardes AM (2018) Recycling waste crystalline silicon photovoltaic modules by electrostatic separation. J Sustain Metall 4(2):176–186
Ministry of the Environment Government of Japan (2016). http://www.env.go.jp/press/files/jp/102441.pdf. (Accessed 20 Jun 2022)
Doi T, Tsuda I, Unagida H, Murata A, Sakuta K, Kurokawa K (2001) Experimental study on PV module recycling with organic solvent. Sol Energy Mater Sol Cells 67(1–4):397–403
New Energy and Industrial Technology Development Organization (NEDO) (2019) https://www.nedo.go.jp/content/100901845.pdf . (Accessed 20 Jun 2022)
Matsubara T, Uddin MA, Kato Y, Kawanishi T, Hayashi Y (2018) Chemical treatment of copper and aluminum derived from waste crystalline silicon solar cell modules by mixed acids of HNO3 and HCl. J Sustain Metall 4:378–387
Takami K, Kobashi M, Shiraga Y, Uddin MA, Kato Y, Wu S (2015) Effect of HF and HNO3 concentration on etching rate of each component in waste crystalline silicon solar cells. Mater Trans 56(12):2047–2052
Klugmann-Radziemska E, Ostrowski P (2010) Chemical treatment of crystalline silicon solar cells as a method of recovering pure silicon from photovoltaic modules. Renew Energ 35(8):1751–1759
Truc NTT, Lee B-K (2016) Sustainable and selective separation of PVC and ABS from a WEEE plastic mixture using microwave and/or mild-heat treatment with froth flotation. Environ Sci Technol 50:10580–10587
Mallampati SR, Lee C-H, Park MH, Lee B-K (2018) Processing plastics from ASR/ESR waste: separation of poly vinyl chloride (PVC) by froth flotation after microwave-assisted surface modification. J Mater Cycles Waste Manag 20:91–99
Qu YH, Li YP, Zou XT, Xu KW, Xue YT (2021) Microwave treatment combined with wetting agent for an efficient flotation separation of acrylonitrile butadiene styrene (ABS). J Mater Cycles Waste Manag 23:96–106
Wang C, Wang H, Fu J, Zhang L, Luo C, Liu Y (2015) Flotation separation of polyvinyl chloride and polyethylene terephthalate plastics combined with surface modification for recycling. Waste Manag 45:112–117
Wang J, Wang H, Wang C, Zhang L, Wang T, Zhang L (2017) A novel process for separation of hazardous poly (vinyl chloride) from mixed plastic wastes by froth flotation. Waste Manag 69:59–65
Li W, Li Y (2022) Selective flotation separation of polycarbonate from plastic mixtures based on Fenton treatment combined with ultrasonic. J Mater Cycles Waste Manag 24:917–926
Ito M, Takeuchi M, Saito A, Murase N, Phengsaart T, Tabelin CB, Hiroyosshi N, Tsunekawa M (2019) Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J Mater Cycles Waste Manag 21:1376–1383
Ito M, Saito A, Murase N, Phengsaart T, Kimura S, Kitajima N, Takeuchi M, Tabelin CB, Hiroyosshi N (2020) Estimation of hybrid jig separation efficiency using a modified concentration criterion based on apparent densities of plastic particles with attached bubbles. J Mater Cycles Waste Manag 20:2071–2080
Jiang H, Zhang Y, Bian K, Wang H, Wang C (2022) Insight into the effects of aqueous species on microplastics removal by froth flotation: kinetics and mechanism. J Environ Chem Eng 10:107834
Zhang Y, Jiang H, Bian K, Wang H, Wang C (2021) A critical review of control and removal strategies for microplastics from aquatic environments. J Environ Chem Eng 9:105463
Eivazihollagh A, Tejera J, Svanedal I, Edlund H, Blanco A (2017) Removal of Cd2+, Zn2+, and Sr2+ by ion flotation, using a surface-active derivative of DTPA (C12-DTPA). Ind Eng Chem Res 56:10605–10614
Cao D, Xu X, Jiang S (2021) Ultrasound-electrochemistry enhanced flotation and desulfurization for fine coal. Sep Purif Technol 258:117968
Jatav PP, Tajane SP, Mandavgane SA, Gaidhani SB (2019) A process of carbon enrichment of bottom slag ash for value-added applications. J Mater Cycles Waste Manag 21:539–546
Xie Q, Wang D, Han Z, Tao H, Liu S (2022) Removal of carbon and dioxins from municipal solid waste incineration fly ash by ball milling and flotation methods. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-022-01514-6
Altansukh B, Burmaa G, Nyamdelger S, Ariunbolor N, Shibayama A, Haga K (2014) Gold recovery from its flotation concentrate using acidic thiourea leaching and organosilicon polymer. Int J Soc Mater Eng Resour 20:29–34
Burat F, Demirag A, Safak MC (2020) Recovery of noble metals from floor sweeping jewelry waste by flotation-cyanide leaching. J Mater Cycles Waste Manag 22:907–915
Dinc NI, Tosun AU, Basturkcu OM, Burat F (2022) Recovery of valuable metals from WPCB fines by centrifugal gravity separation and froth flotation. J Mater Cycles Waste Manag 24:224–236
Harada S, Uddin MA, Kato Y, Kawanishi T, Hayashi Y (2019) Separation between silicon and aluminum powders contained within pulverized scraped silicon-based waste solar cells by flotation method. J Sustain Metall 5:551–560
Park C-H, Subasinghe N, Han O-H (2015) Amenability testing of fine coal beneficiation using laboratory flotation column. Mater Trans 56:766–773
Vinett L, Waters KE (2020) Representation of kinetics models in batch flotation as distributed first-order reactions. Minerals 10:913
Matsuoka H, Mitsuhashi K, Kawata M, Tokoro C (2020) Deviation of flotation kinetic model for activated and depresses copper sulfide minerals. Minerals 10:1027
Javanovic I, Miljanovic I (2015) Modelling of flotation processes by classical mathematical methods- a review. Arch Min Sci 60:905–919
Gharai M, Venugopal R (2016) Modeling of flotation process – an overview of different approaches. Miner Process Extr Metall Rev 37:120–133
Wang L, Peng Y, Runge K, Bradshaw D (2015) A review of entrainment: mechanisms, contributing factors and modelling in flotation. Miner Eng 70:77–91
Imaizumi T, Inoue T (1961) A study of flotation as a rate process. J Min Metall Inst Jpn 77:987–994
Nguyen AV, Harvey PA, Jameson GJ (2003) Influence of gas flow rate and frothers on water recovery in a froth column. Miner Eng 16:1143–1147
Takamori T, Fukami S (1970) New method for evaluation of the flotation characteristics of ore. Flotation 41:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kato, Y., Harada, S., Nishimura, N. et al. Flotation kinetics of aluminum powders derived from waste crystalline silicon solar cells and its comparison between batch, continuous and column flotation practices. J Mater Cycles Waste Manag 25, 826–834 (2023). https://doi.org/10.1007/s10163-022-01564-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01564-w