Abstract
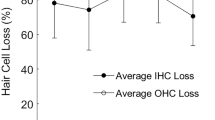
Integration of acoustic information over time is essential for processing complex stimuli, such as speech, due to its continuous variability along the time domain. In both humans and animals, perception of acoustic stimuli is a function of both stimulus intensity and duration. For brief acoustic stimuli, as duration increases, thresholds decrease by approximately 3 dB for every doubling in duration until stimulus duration reaches 500 ms, a phenomenon known as temporal integration. Although hearing loss and damage to outer hair cells (OHC) have been shown to alter temporal integration in some studies, the role of cochlear inner hair cells (IHC) on temporal integration is unknown. Because IHC transmit nearly all acoustic information to the central auditory system and are believed to code both intensity and timing information, these sensory cells likely play a critical role in temporal integration. To test the hypothesis that selective IHC loss degrades the temporal integration function, behaviorally trained chinchillas were treated with carboplatin, a drug known to selectively destroy IHC with little to no effect on OHC in this species. Pure-tone thresholds were assessed across frequencies (1, 2, 4, 8, 12 kHz) as a function of signal duration (500, 100, 50, 10, and 5 ms). Baseline testing showed a significant effect of duration on thresholds. Threshold decreased as a function of increasing duration, as expected. Carboplatin treatment (75 mg/kg) produced a moderate to severe loss of IHC (45–85%) with little-to-no loss of OHC. Contrary to our hypothesis, post-carboplatin temporal integration thresholds showed no significant differences from baseline regardless of stimulus duration or frequency. These data suggest that few IHC are necessary for temporal integration of simple stimuli. Temporal integration may be sensitive to loss of OHC and loss of cochlear non-linearities but does not appear to be sensitive to selective IHC loss.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- ANF,:
-
Auditory nerve fibers
- CL:
-
Cochlear hearing loss
- dB:
-
Decibel
- IHC:
-
Inner hair cell
- NH:
-
Normal hearing
- OHC:
-
Outer hair cell
- SPL:
-
Sound pressure level
References
Allen JB (1980) Cochlear micromechanics–a physical model of transduction. J Acoust Soc Am 68:1660–1670
Badri R, Siegel JH, Wright BA (2011) Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J Acoust Soc Am 129:852–863
Blakeslee EA, Hynson K, Hamernik RP, Henderson D (1978) Asymptotic threshold shift in chinchillas exposed to impulse noise. J Acoust Soc Am 63:876–882
Borg E (1987) Loss of hair cells and threshold sensitivity during prolonged noise exposure in normotensive albino rats. Hear Res 30:119–126
Buus S, Florentine M, Poulsen T (1999) Temporal integration of loudness in listeners with hearing losses of primarily cochlear origin. J Acoust Soc Am 105:3464–3480
Carlyon RP, Buus S, Florentine M (1990) Temporal integration of trains of tone pulses by normal and by cochlearly impaired listeners. J Acoust Soc Am 87:260–268
Chiu F, Rakusen LL, Mattys SL (2019) Cognitive load elevates discrimination thresholds of duration, intensity, and f0 for a synthesized vowel. J Acoust Soc Am 146:1077
Chung DY (1981) Tone-on-tone masking in subjects with normal hearing and with sensorineural hearing loss. J Speech Hear Res 24:506–513
Cody AR, Russell IJ (1985) Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature 315:662–665
Dallos PJ, Johnson KR (1966) Influence of rise-fall time upon short-tone threshold. J Acoust Soc Am 40:1160–1163
Davis B, Qiu W, Hamernik RP (2005) Sensitivity of distortion product otoacoustic emissions in noise-exposed chinchillas. J Am Acad Audiol 16:69–78
Davis RI, Ahroon WA, Hamernik RP (1989) The relation among hearing loss, sensory cell loss and tuning characteristics in the chinchilla. Hear Res 41:1–14
DeFilippo CL, Snell KB (1986) Detection of a temporal gap in low-frequency narrow-band signals by normal-hearing and hearing-impaired listeners. J Acoust Soc Am 80:1354–1358
Eddins AC, Salvi RJ, Wang J, Powers NL (1998) Threshold-duration functions of chinchilla auditory nerve fibers. Hear Res 124:190
Elliott LL (1975) Temporal and masking phenomena in persons with sensorineural hearing loss. Audiology: official organ of the Int Soc Audio14:336–353
Fettiplace R, Fuchs PA (1999) Mechanisms of hair cell tuning. Annu Rev Physiol 61:809–834
Fitzgibbons PJ, Wightman FL (1982) Gap detection in normal and hearing-impaired listeners. J Acoust Soc Am 72:761–765
Florentine M, Buus S (1984) Temporal gap detection in sensorineural and simulated hearing impairments. J Speech Hear Res 27:449–455
Florentine M, Fastl H, Buus S (1988) Temporal integration in normal hearing, cochlear impairment, and impairment simulated by masking. J Acoust Soc Am 84:195–203
Gengel RW, Watson CS (1971) Temporal integration. I. Clinical implications of a laboratory study. II. Additional data from hearing-impaired subjects. J Speech Hear Disord 36:213–224
Gerken GM, Bhat VK, Hutchison-Clutter M (1990) Auditory temporal integration and the power function model. J Acoust Soc Am 88:767–778
Giraudi-Perry DM, Salvi RJ, Henderson D (1982) Gap detection in hearing-impaired chinchillas. J Acoust Soc Am 72:1387–1393
Giraudi D, Salvi R, Henderson D, Hamernik R (1980) Gap detection by the chinchilla. J Acoust Soc Am 68:802–806
Gleich O, Kittel MC, Klump GM, Strutz J (2007) Temporal integration in the gerbil: the effects of age, hearing loss and temporally unmodulated and modulated speech-like masker noises. Hear Res 224:101–114
Gordon-Salant S, Fitzgibbons PJ (2001) Sources of age-related recognition difficulty for time-compressed speech. Journal of Speech, Language, and Hearing Research : JSLHR 44:709–719
Gordon-Salant S, Fitzgibbons PJ, Friedman SA (2007) Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. Journal of Speech, Language, and Hearing Research : JSLHR 50:1181–1193
Gorga MP, Beauchaine KA, Reiland JK, Worthington DW, Javel E (1984) The effects of stimulus duration on ABR and behavioral thresholds. J Acoust Soc Am 76:616–619
Grose JH, Eddins DA, Hall JW 3rd (1989) Gap detection as a function of stimulus bandwidth with fixed high-frequency cutoff in normal-hearing and hearing-impaired listeners. J Acoust Soc Am 86:1747–1755
Hall JW, Fernandes MA (1983) Temporal integration, frequency resolution, and off-frequency listening in normal-hearing and cochlear-impaired listeners. J Acoust Soc Am 74:1172–1177
Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA (1989) The quantitative relation between sensory cell loss and hearing thresholds. Hear Res 38:199–211
Henderson D (1969) Temporal summation of acoustic signals by the chinchilla. J Acoust Soc Am 46:474–475
Hofstetter P, Ding D, Salvi R (1997a) Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology 36:301–311
Hofstetter P, Ding D, Powers N, Salvi RJ (1997b) Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res 112:199–215
Janesick A, Scheibinger M, Benkafadar N, Kirti S, Ellwanger DC, Heller S (2021) Cell-type identity of the avian cochlea. Cell Rep 34:108900
Johnstone BM, Patuzzi R, Yates GK (1986) Basilar membrane measurements and the travelling wave. Hear Res 22:147–153
King KA, Gordon-Salant S, Pawlowski KS, Taylor AM, Griffith AJ, Houser A, Kurima K, Wassif CA, Wright CG, Porter FD, Repa JJ, Brewer CC (2014) Hearing loss is an early consequence of Npc1 gene deletion in the mouse model of Niemann-Pick disease, type C. Journal of the Association for Research in Otolaryngology : JARO 15:529–541
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after "temporary" noiseinduced hearing loss. The Journal of neuroscience: the official journal of the Society for Neuroscience 29:14077–14085
Lauer AM, Dooling RJ, Leek MR, Poling K (2007) Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. J Acoust Soc Am 122:3615–3627
Lobarinas E, Salvi R, Ding D (2013) Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res 302:113–120
Lobarinas E, Salvi R, Ding D (2016) Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. Journal of the Association for Research in Otolaryngology : JARO 17:89–101
Lobarinas E, Salvi R, Ding D (2020) Gap detection deficits in chinchillas with selective carboplatin-induced inner hair cell loss. J Assoc Res Otolaryngol: JARO 21(6):475–483
Manheim M, Lavie L, Banai K (2018) Age, hearing, and the perceptual learning of rapid speech. Trends Hear 22:2331216518778651
McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ (2002) Chinchilla models of selective cochlear hair cell loss. Hear Res 174:230–238
Middelweerd MJ, Festen JM, Plomp R (1990) Difficulties with speech intelligibility in noise in spite of a normal pure-tone audiogram. Audiology: official organ of the Int Soc Audio 29:1–7
Moore BC, Peters RW, Glasberg BR (1992) Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss. J Acoust Soc Am 92:1923–1932
Moore BC, Peters RW, Glasberg BR (1999) Effects of frequency and duration on psychometric functions for detection of increments and decrements in sinusoids in noise. J Acoust Soc Am 106:3539–3552
Muller M, Hoidis S, Smolders JW (2010) A physiological frequency-position map of the chinchilla cochlea. Hear Res 268:184–193
Neubauer H, Heil P (2004) Towards a unifying basis of auditory thresholds: the effects of hearing loss on temporal integration reconsidered. Journal of the Association for Research in Otolaryngology : JARO 5:436–458
Oxenham AJ, Moore BC, Vickers DA (1997) Short-term temporal integration: evidence for the influence of peripheral compression. J Acoust Soc Am 101:3676–3687
Palandrani KN, Hoover EC, Stavropoulos T, Seitz AR, Isarangura S, Gallun FJ, Eddins DA (2021) Temporal integration of monaural and dichotic frequency modulation. J Acoust Soc Am 150:745
Patuzzi RB, Yates GK, Johnstone BM (1989) Outer hair cell receptor current and sensorineural hearing loss. Hear Res 42:47–72
Pawlowski K, Koulich E, Wright CG, Roland P (2013) Ototopic applications of povidone iodine/dexamethasone in the rat. Otology & neurotology : official publication of the American Otological Society, Am Neurotol Soc Eur Academy Otol Neurotol 34:167–174
Pedersen CB, Salomon G (1977) Temporal integration of acoustic energy. Acta Otolaryngol 83:417–423
Preyer S, Gummer AW (1996) Nonlinearity of mechanoelectrical transduction of outer hair cells as the source of nonlinear basilar-membrane motion and loudness recruitment. Audiol Neurootol 1:3–11
Reed CM, Braida LD, Zurek PM (2009) Review article: review of the literature on temporal resolution in listeners with cochlear hearing impairment: a critical assessment of the role of suprathreshold deficits. Trends Amplif 13:4–43
Salvi RJ, Arehole S (1985) Gap detection in chinchillas with temporary high-frequency hearing loss. J Acoust Soc Am 77:1173–1177
Salvi RJ, Hamernik RP, Henderson D (1978) Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Exp Brain Res 32:301–320
Saunders SS, Salvi RJ, Miller KM (1995) Recovery of thresholds and temporal integration in adult chickens after high-level 525-Hz pure-tone exposure. J Acoust Soc Am 97:1150–1164
Solecki JM, Gerken GM (1990) Auditory temporal integration in the normal-hearing and hearing-impaired cat. J Acoust Soc Am 88:779–785
Song Q, Shen P, Li X, Shi L, Liu L, Wang J, Yu Z, Stephen K, Aiken S, Yin S, Wang J (2016) Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Scientific reports 6:25200
Spoendlin H (1975) Neuroanatomical basis of cochlear coding mechanisms. Audiology 14:383–407
Stephens SD (1973) Auditory temporal integration as a function of intensity. J Sound Vib 30:109–126
Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ (1994a) Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res 75:93–102
Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y (1994b) Induction of selective inner hair cell damage by carboplatin. Scanning Microsc 8:97–106
Thibodeau LM (1996) Evaluation of auditory enhancement and auditory suppression in listeners with normal hearing and reduced speech recognition in noise. J Speech Hear Res 39:947–956
Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A (1996) Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res 96:71–82
Trevino M, Lobarinas E, Maulden AC, Heinz MG (2019) The chinchilla animal model for hearing science and noise-induced hearing loss. J Acoust Soc Am 146:3710
Turner CW, Robb MP (1987) Audibility and recognition of stop consonants in normal and hearing-impaired subjects. J Acoust Soc Am 81:1566–1573
Viemeister NF, Wakefield GH (1991) Temporal integration and multiple looks. J Acoust Soc Am 90:858–865
Wake M, Takeno S, Ibrahim D, Harrison R (1994) Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope 104:488–493
Walden BE, Schwartz DM, Montgomery AA, Prosek RA (1981) A comparison of the effects of hearing impairment and acoustic filtering on consonant recognition. J Speech Hear Res 24:32–43
Watson CS, Gengel RW (1969) Signal duration and signal frequency in relation to auditory sesitivity. J Acoust Soc Am 46:989–997
Wever EG, Bray CW (1930) Auditory nerve impulses. Science 71:215
Author information
Authors and Affiliations
Contributions
All authors contributed to data collection and writing of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trevino, M., Escabi, C.D., Zang, A. et al. Effect of Selective Carboplatin-Induced Inner Hair Cell Loss on Temporal Integration in Chinchillas. JARO 23, 379–389 (2022). https://doi.org/10.1007/s10162-022-00843-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-022-00843-5