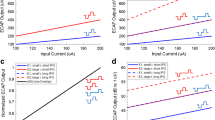
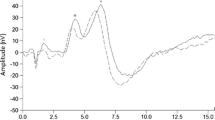
Abstract
Several physiological mechanisms act on the response of the auditory nerve (AN) during acoustic stimulation, resulting in an adjustment in auditory gain. These mechanisms include—but are not limited to—firing rate adaptation, dynamic range adaptation, the middle ear muscle reflex, and the medial olivocochlear reflex. A potential role of these mechanisms is to improve the neural signal-to-noise ratio (SNR) at the output of the AN in real time. This study tested the hypothesis that neural SNRs, inferred from non-invasive assessment of the human AN, improve over the duration of acoustic stimulation. Cochlear potentials were measured in response to a series of six high-level clicks embedded in a series of six lower-level broadband noise bursts. This paradigm elicited a compound action potential (CAP) in response to each click and to the onset of each noise burst. The ratio of CAP amplitudes elicited by each click and noise burst pair (i.e., neural SNR) was tracked over the six click/noise bursts. The main finding was a rapid (< 24 ms) increase in neural SNR from the first to the second click/noise burst, consistent with a real-time adjustment in the response of the auditory periphery toward improving the SNR of the signal transmitted to the brainstem. Analysis of cochlear microphonic and ear canal sound pressure recordings, as well as the time course for this improvement in neural SNR, supports the conclusion that firing rate adaptation is likely the primary mechanism responsible for improving neural SNR, while dynamic range adaptation, the middle ear muscle reflex, and the medial olivocochlear reflex played a secondary role on the effects observed in this study. Real-time improvements in neural SNR are significant because they may be essential for robust encoding of speech and other relevant stimuli in the presence of background noise.






Similar content being viewed by others
Notes
An artifact created by the probe microphone/tube assembly occurred for the first burst, resulting in a spectral difference between the first burst compared to the remaining bursts. This spectral difference was identified as an artifact, rather than of biological origin, by conducting probe microphone measurements in a syringe with the probe microphone tubing position, foam ear tip depth, and syringe volume set to approximate those associated with measurement in the human ear canal. Sound pressure measurements in the syringe resulted in a constant spectral difference between the first burst and the remaining bursts, similar to that observed in ear canal measurements of human participants. The spectral configuration of the artifact varied among participants, consistent with individual differences in outer and middle ear anatomy and probe insertion depth. Thus, the use of the second burst as the reference provides a custom correction factor for each participant. Note that this approach should have little to no impact on assessing the effects of the MEM reflex on neural SNR given that MEM activity is not expected for the 24 ms between the first and second burst, as the time constant for the MEM reflex is roughly 50 ms (Hung and Dallos 1972).
References
Backus BC, Guinan JJ (2006) Time-course of the human medial olivocochlear reflex. J Acoust Soc Am 119:2889–2904
Cheatham MA, Naik K, Dallos P (2011) Using the cochlear microphonic as a tool to evaluate cochlear function in mouse models of hearing. J Assoc Res Otolaryngol 12:113–125
Chertoff ME, Earl BR, Diaz FJ, Sorensen JL, Thomas ML, Kamerer AM, Peppi M (2014) Predicting the location of missing outer hair cells using the electrical signal recorded at the round window. J Acoust Soc Am 136:1212
Cooper NP, Guinan JJ (2006) Efferent-mediated control of basilar membrane motion. J Physiol 576:49–54
Dean I, Harper NS, McAlpine D (2005) Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8:1684–1689
Eggermont J, Odenthal D (1974) Electrophysiological investigation of the human cochlea: recruitment, masking and adaptation. Audiology 13:1–22
Feeney MP, Keefe DH (2001) Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power. Ear Hear 22:316–332
Fex J (1959) Augmentation of cochlear microphonic by stimulation of efferent fibres to the cochlea; preliminary report. Acta Otolaryngol 50:540–541
Fex J (1967) Efferent inhibition in the cochlea related to hair-cell dc activity: study of postsynaptic activity of the crossed olivocochlear fibres in the cat. J Acoust Soc Am 41:666–675
Folsom RC, Owsley RM (1987) N1 action potentials in humans: Influence of simultaneous contralateral stimulation. Acta Otolaryngol 103:262–265
Guinan JJ (1996) Physiology of the Olivocochlear Efferents. In: Dallos P, Popper AN, Fay RR (eds) The Cochlea. Springer, New York, pp 435–502
Henry KR (1995) Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear Res 90:176–184
Hung IJ, Dallos P (1972) Study of the acoustic reflex in human beings. I. Dynamic Characteristics J Acoust Soc Am 52:1168–1180
Jamos AM, Kaf WA, Chertoff ME, Ferraro JA (2020) Human medial olivocochlear reflex: Contralateral activation effect on low and high frequency cochlear response. Hear Res 389:107925
Jamos AM, Chertoff ME, Kaf WA, Ferraro JA (2021a) Medial Olivocochlear Reflex Effect on Cochlear Response in Humans: Elicitor Side and Level. J Am Acad Audiol 32:366–373
Jamos AM, Hosier B, Davis S, Franklin TC (2021b) The Role of the Medial Olivocochlear Reflex in Acceptable Noise Level in Adults. J Am Acad Audiol 32:137–143
Jennings SG (2021) The role of the medial olivocochlear reflex in psychophysical masking and intensity resolution in humans: a review. J Neurophysiol 125:2279–2308
Kawase T, Liberman MC (1993) Antimasking Effects of the Olivocochlear Reflex. 1. Enhancement of Compound Action-Potentials to Masked Tones. J Neurophysiol 70:2519–2532
Kawase T, Takasaka T (1995) The effects of contralateral noise on masked compound action potential in humans. Hear Res 91:1–6
Kawase T, Delgutte B, Liberman MC (1993) Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70:2533–2549
Kiang NYS (1965) Discharge patterns of single fibers in the cat's auditory nerve. Cambridge, Mass.,: M.I.T. Press
Liberman MC, Guinan JJ (1998) Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J Commun Disord 31:471–482
Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419:300–304
Lichtenhan JT, Cooper NP, Guinan JJ Jr (2013) A new auditory threshold estimation technique for low frequencies: proof of concept. Ear Hear 34:42–51
Lichtenhan JT, Wilson US, Hancock KE, Guinan JJ Jr (2016) Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses. Hear Res 333:216–224
Lopez-Poveda EA (2018) Olivocochlear Efferents in Animals and Humans: From Anatomy to Clinical Relevance. Front Neurol 9:197
Margolis RH (1999) Electrocochleography. Semin Hear 45–60. Copyright© 1999 by Thieme Medical Publishers, Inc
Moller AR (1965) An Experimental Study of the Acoustic Impedance of the Middle Ear and Its Transmission Properties. Acta Otolaryngol 60:129–149
Møller AR (1962) Acoustic reflex in man. J Acoust Soc Am 34:1524–1534
Nieder P, Nieder I (1970) Antimasking effect of crossed olivocochlear bundle stimulation with loud clicks in guinea pig. Exp Neurol 28:179–188
Pilz PK, Ostwald J, Kreiter A, Schnitzler HU (1997) Effect of the middle ear reflex on sound transmission to the inner ear of rat. Hear Res 105:171–182
Rabbitt RD, Clifford S, Breneman KD, Farrell B, Brownell WE (2009) Power efficiency of outer hair cell somatic electromotility. PLoS Comput Biol 5:e1000444
Robinson BL, McAlpine D (2009) Gain control mechanisms in the auditory pathway. Curr Opin Neurobiol 19:402–407
Robles L, Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81:1305–1352
Ruggero MA, Robles L, Rich NC (1992) Two-tone suppression in the basilar membrane of the cochlea: mechanical basis of auditory-nerve rate suppression. J Neurophysiol 68:1087–1099
Ruggero MA, Rich NC, Recio A, Narayan SS, Robles L (1997) Basilar-membrane responses to tones at the base of the chinchilla cochlea. J Acoust Soc Am 101:2151–2163
Schoonhoven R, Fabius MA, Grote JJ (1995) Input/output curves to tone bursts and clicks in extratympanic and transtympanic electrocochleography. Ear Hear 16:619–630
Simpson MJ, Jennings SG, Margolis RH (2020) Techniques for Obtaining High-quality Recordings in Electrocochleography. Front Sys Neurosci 14:18
Smith RL, Zwislocki JJ (1975) Short-term adaptation and incremental responses of single auditory-nerve fibers. Biol Cybern 17:169–182
Smith SB, Lichtenhan JT, Cone BK (2017) Contralateral Inhibition of Click- and Chirp-Evoked Human Compound Action Potentials. Front Neurosci 11:189
Wen B, Wang GI, Dean I, Delgutte B (2009) Dynamic range adaptation to sound level statistics in the auditory nerve. J Neurosci 29:13797–13808
Wen B, Wang GI, Dean I, Delgutte B (2012) Time course of dynamic range adaptation in the auditory nerve. J Neurophysiol 108:69–82
Westerman LA, Smith RL (1984) Rapid and short-term adaptation in auditory nerve responses. Hear Res 15:249–260
Wojtczak M, Beim JA, Oxenham AJ (2017) Weak Middle-Ear-Muscle Reflex in Humans with Noise-Induced Tinnitus and Normal Hearing May Reflect Cochlear Synaptopathy. eNeuro 4
Acknowledgements
This work was supported by grant K23 DC014752 from NIH/NIDCD (PI: Jennings).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jennings, S.G., Dominguez, J. Firing Rate Adaptation of the Human Auditory Nerve Optimizes Neural Signal-to-Noise Ratios. JARO 23, 365–378 (2022). https://doi.org/10.1007/s10162-022-00841-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-022-00841-7