Abstract
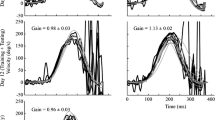
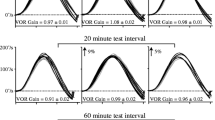
The vestibulo-ocular reflex (VOR) is the main retinal image stabilising mechanism during rapid head movement. When the VOR does not stabilise the world or target image on the retina, retinal image slip occurs generating an error signal that drives the VOR response to increase or decrease until image slip is minimised, i.e. VOR adaptation occurs. Visual target contrast affects the human smooth pursuit and optokinetic reflex responses. We sought to determine if contrast also affected VOR adaptation. We tested 12 normal subjects, each over 16 separate sessions. For sessions 1–14, the ambient light level (lx) during adaptation training was as follows: dark, 0.1, 0.2, 0.3, 0.5, 0.7, 1, 2, 8, 16, 32, 64, 128 and 255 lx (light level for a typical room). For sessions 15–16, the laser target power (related to brightness) was halved with ambient light at 0 and 0.1 lx. The adaptation training lasted 15 min and consisted of left/right active head impulses. The VOR gain was challenged to increment, starting at unity, by 0.1 every 90 s for rotations to the designated adapting side and fixed at unity towards the non-adapting side. We measured active and passive VOR gains before and after adaptation training. We found that for both the active and passive VOR, there was a significant increase in gain only towards the adapting side due to training at contrast level 1.5 k and above (2 lx and below). At contrast level 261 and below (16 lx and above), adaptation training resulted in no difference between adapting and non-adapting side gains. Our modelling suggests that a contrast threshold of ~ 1000, which is 60 times higher than that provided by typical room lighting, must be surpassed for robust active and passive VOR adaptation. Our findings suggest contrast is an important factor for adaptation, which has implication for rehabilitation programs.




Similar content being viewed by others
References
Bartl K, Lehnen N, Kohlbecher S, Schneider E (2009) Head impulse testing using video-oculography. Ann N Y Acad Sci 1164:331–333
Büttner U, Büttner-Ennever JA (2006) Present concepts of oculomotor organization. Prog Brain Res 151:1–42
Cohen B, Henn V, Raphan T, Dennett D (1981) Velocity storage, nystagmus, and visual-vestibular interactions in humans. Ann N Y Acad Sci 374:421–433
Conrad, J. (2003) Exposure metering: relating subject lighting to film exposure. Available online:http://www.largeformatphotography.info/articles/conrad-meter-cal.pdf (accessed on 18 January 2017)
Fadaee SB, Migliaccio AA (2016) The effect of retinal image error update rate on human vestibule-ocular reflex gain adaptation. Exp Brain Res 234:1085–1094
Gauthier GM, Robinson DA (1975) Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Res 92:331–335
Gonshor A, Jones GMJ (1976) Short-term adaptive changes in the human vestibulo-ocular reflex arc. Physiol 256:361–379
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45:737–9
Hiscocks, P.D. (2014) Measuring luminance with a digital camera. Available online: http://www.ee.ryerson.ca/~phiscock/astronomy/light-pollution/luminance-notes-2.pdf (accessed on 18 January 2017)
Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65:170–174
Kingdom FA (2011) Lightness, brightness and transparency: a quarter century of new ideas, captivating demonstrations and unrelenting controversy. Vis Res 51:652–673
Leguire LE, Zaff BS, Freeman S, Rogers GL, Bremer DL (1991) Contrast sensitivity of optokinetic nystagmus. Vis Res 31:89–97
Luebke AE, Robinson DA (1994) Gain changes of the cat’s vestibulo-ocular reflex after flocculus deactivation. Exp Brain Res 98:379–390
Madgwick SO, Harrison AJ, Vaidyanathan A. (2011) Estimation of IMU and MARG orientation using a gradient descent algorithm. IEEE International Conference on Rehabilitation Robotics, pp. 1–7
Migliaccio AA, Schubert MC (2013) Unilateral adaptation of the human angular vestibulo-ocular reflex. J Assoc Res Otolaryngol 14:29–36
Migliaccio AA, Schubert MC (2014) Pilot study of a new rehabilitation tool: improved unilateral short-term adaptation of the human angular vestibulo-ocular reflex. Otol Neurotol 35:310–316
Schubert MC, Della Santina CC, Shelhamer M (2008) Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res 191:435–446
Spering M, Kerzel D, Braun DI, Hawken MJ, Gegenfurtner KR (2005) Effects of contrast on smooth pursuit eye movements. J Vis 5:455–465. https://doi.org/10.1167/5.5.6
Spoor M, Hosseini B, van Alphen B, Frens MA, van der Geest JN (2014) Human gaze following response is affected by visual acuity. J Ophthalmol 2014:543478. https://doi.org/10.1155/2014/543478
Sumnall JH, Freeman TC, Snowden RJ (2003) Optokinetic potential and the perception of head-centred speed. Vis Res 43:1709–1718
Thompson P (1982) Perceived rate of movement depends on contrast. Vis Res 22:377–380
Thompson P (1983) Discrimination of moving gratings at and above detection threshold. Vis Res 23:1533–1538
Waddington J, Harris CM (2015) Human optokinetic nystagmus and spatial frequency. J Vis 15:7. https://doi.org/10.1167/15.13.7
Weber KP, Aw ST, Todd MJ, McGarvie LA, Pratap S, Curthoys IS, Halmagyi GM (2008) Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing. Prog Brain Res 171:195–198
Whittle P (1994) The psychophysics of contrast brightness. In: Gilchrist AL (ed) Lightness, brightness, and transparency. Lawrence Erlbaum Associates, Hillsdale, NJ, pp 35–110
Zee DS, Yamazaki A, Butler PH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 46:878–899
Acknowledgements
A.A. Migliaccio was supported by The Garnett Passe and Rodney Williams Memorial Foundation Senior/Principal Research Fellowship in Otorhinolaryngology and Project Grant (2013-15), and NHMRC Development Grant APP105550.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muntaseer Mahfuz, M., Schubert, M.C., Todd, C.J. et al. The Effect of Visual Contrast on Human Vestibulo-Ocular Reflex Adaptation. JARO 19, 113–122 (2018). https://doi.org/10.1007/s10162-017-0644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0644-6