Abstract
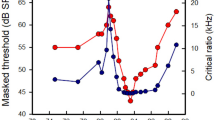
The auditory brainstem response (ABR) is an evoked potential that reflects the responses to sound by brainstem neural centers. The binaural interaction component (BIC) is obtained by subtracting the sum of the monaural ABR responses from the binaural response. Its latency and amplitude change in response to variations in binaural cues. The BIC is thus thought to reflect the activity of binaural nuclei and is used to non-invasively test binaural processing. However, any conclusions are limited by a lack of knowledge of the relevant processes at the level of individual neurons. The aim of this study was to characterize the ABR and BIC in the barn owl, an animal where the ITD-processing neural circuits are known in great detail. We recorded ABR responses to chirps and to 1 and 4 kHz tones from anesthetized barn owls. General characteristics of the barn owl ABR were similar to those observed in other bird species. The most prominent peak of the BIC was associated with nucleus laminaris and is thus likely to reflect the known processes of ITD computation in this nucleus. However, the properties of the BIC were very similar to previously published mammalian data and did not reveal any specific diagnostic features. For example, the polarity of the BIC was negative, which indicates a smaller response to binaural stimulation than predicted by the sum of monaural responses. This is contrary to previous predictions for an excitatory-excitatory system such as nucleus laminaris. Similarly, the change in BIC latency with varying ITD was not distinguishable from mammalian data. Contrary to previous predictions, this behavior appears unrelated to the known underlying neural delay-line circuitry. In conclusion, the generation of the BIC is currently inadequately understood and common assumptions about the BIC need to be reconsidered when interpreting such measurements.









Similar content being viewed by others
References
Beutelmann R, Laumen G, Tollin D, Klump GM (2015) Amplitude and phase equalization of stimuli for click evoked auditory brainstem responses. J Acoust Soc Am 137:EL71–EL77. doi:10.1121/1.4903921
Brand A, Behrend O, Marquardt T, et al. (2002) Precise inhibition is essential for microsecond interaural time difference coding. Nature 417:543–547. doi:10.1038/417543a
Brittan-Powell EF, Dooling RJ, Gleich O (2002) Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus). J Acoust Soc Am 112:999. doi:10.1121/1.1494807
Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ (2005) Auditory brainstem responses in the eastern screech owl: an estimate of auditory thresholds. J Acoust Soc Am 118:314. doi:10.1121/1.1928767
Calford MB, Piddington RW (1988) Avian interaural canal enhances interaural delay. J Comp Physiol A 162:503–510. doi:10.1007/BF00612515
Calvo C, Moiseff A (1992) Neural correlates of interaural time processing in the auditory evoked potentials of the barn owl. Soc Neurosci Abstr 18:149
Calvo C, Moiseff A (1993) Monitoring of the binaural interaction in the immature barn owl. Soc Neurosci Abstr 19:531
Carr CE, Konishi M (1990) A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10:3227–3246
Carr CE, Köppl C (2004) Coding interaural time differences at low best frequencies in the barn owl. J Physiol 98:99–112. doi:10.1016/j.jphysparis.2004.03.003
Dobie RA, Berlin CI (1979) Binaural interaction in brainstem-evoked responses. Arch Otolaryngol - Head Neck Surg 105:391–398. doi:10.1001/archotol.1979.00790190017004
Fobel O, Dau T (2004) Searching for the optimal stimulus eliciting auditory brainstem responses in humans. J Acoust Soc Am 116:2213–2222. doi:10.1121/1.1787523
Furst M, Eyal S, Korczyn AD (1990) Prediction of binaural click lateralization by brainstem auditory evoked potentials. Hear Res 49:347–359. doi:10.1016/0378-5955(90)90113-4
Gaumond RP, Psaltikidou M (1991) Models for the generation of the binaural difference response. J Acoust Soc Am 89:454–456
Goksoy C, Demirtas S, Yagcioglu S, Ungan P (2005) Interaural delay-dependent changes in the binaural interaction component of the Guinea pig brainstem responses. Brain Res 1054:183–191. doi:10.1016/j.brainres.2005.06.083
Goldberg JM, Brown PB (1968) Functional organization of the dog superior olivary complex: an anatomical and electrophysiological study. J Neurophysiol 31:639–656
Goldberg JM, Brown PB (1969) Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32:613–636
Goldwyn JH, Mc Laughlin M, Verschooten E, et al. (2014) A model of the medial superior olive explains spatiotemporal features of local field potentials. J Neurosci 34:11705–11722. doi:10.1523/JNEUROSCI.0175-14.2014
Granzow M, Riedel H, Kollmeier B (2001) Single-sweep-bades methods to improve the quality of auditory brain stem responses part I: optimized linear filtering. Z Audiol 40:32–44
Grothe B (2000) The evolution of temporal processing in the medial superior olive, an auditory brainstem structure. Prog Neurobiol 61:581–610
Grothe B, Pecka M (2014) The natural history of sound localization in mammals: a story of neuronal inhibition. Front Neural Circuits 8:1–19. doi:10.3389/fncir.2014.00116
Grothe B, Pecka M, McAlpine D (2010) Mechanisms of sound localization in mammals. Physiol Rev 90:983–1012. doi:10.1152/physrev.00026.2009
Huang CM, Buchwald JS (1978) Factors that affect the amplitudes and latencies of the vertex short latency acoustic responses in the cat. Electroencephalogr Clin Neurophysiol 44:179–186
Jeffress LA (1948) A place theory of sound localization. J Comp Physiol Psychol 41:35–39
Jones SJ, Van der Poel JC (1990) Binaural interaction in the brain-stem auditory evoked potential: evidence for a delay line coincidence detection mechanism. Electroencephalogr Clin Neurophysiol Potentials Sect 77:214–224. doi:10.1016/0168-5597(90)90040-K
Joris PX, Yin TC (1995) Envelope coding in the lateral superior olive. I Sensitivity to interaural time differences J Neurophysiol 73:1043–1062
Karino S, Smith PH, Yin TC, et al. (2011) Axonal branching patterns as sources of delay in the mammalian auditory brainstem: a re-examination. J Neurosci 31:3016–3031
Konishi M (2003) Coding of auditory space. Annu Rev Neurosci 26:31–55. doi:10.1146/annurev.neuro.26.041002.131123
Köppl C (1997a) Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci 17:3312–3321
Köppl C (1997b) Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. J Neurophysiol 77:364–377
Köppl C, Carr CE (2008) Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biol Cybern 98:541–559. doi:10.1007/s00422-008-0220-6
Köppl C, Gleich O (2007) Evoked cochlear potentials in the barn owl. J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol 193:601–612. doi:10.1007/s00359-007-0215-0
Kubke MF, Massoglia DP, Carr CE (2004) Bigger brains or bigger nuclei? Regulating the size of auditory structures in birds. Brain Behav Evol 63:169–180. doi:10.1159/000076242
Kuokkanen PT, Wagner H, Ashida G, et al. (2010) On the origin of the extracellular field potential in the nucleus laminaris of the barn owl (Tyto alba). J Neurophysiol 104:2274–2290. doi:10.1152/jn.00395.2010
Kuokkanen PT, Ashida G, Carr CE, et al. (2013) Linear summation in the barn owl’s brainstem underlies responses to interaural time differences. J Neurophysiol 110:117–130. doi:10.1152/jn.00410.2012
Larsen O, Dooling R, Ryals BM (1997) Roles of intracranial air pressure in bird audition. In: Lewis ER, Long GR, Lyon RF, Narins PM, Steele CR, Hecht-Poinar E (eds) Diversity in auditory mechanics. World Scientific, Singapore, pp. 11–17
Laumen G, Ferber AT, Klump GM, Tollin DJ (2016a) The physiological basis and clinical use of the binaural interaction component of the auditory brainstem response. Ear Hear doi. doi:10.1097/AUD.0000000000000301
Laumen G, Tollin DJ, Beutelmann R, Klump GM (2016b) Aging effects on the binaural interaction component of the auditory brainstem response in the Mongolian gerbil: effects of interaural time and level differences. Hear Res 337:46–58. doi:10.1016/j.heares.2016.04.009
Manley GA, Köppl C, Konishi M (1988) A neural map of interaural intensity differences in the brain stem of the barn owl. J Neurosci 8:2665–2676
McColgan T, Shah S, Koppl C, et al. (2014) A functional circuit model of interaural time difference processing. J Neurophysiol 112:2850–2864. doi:10.1152/jn.00484.2014
McPherson DL, Starr A (1993) Binaural interaction in auditory evoked potentials: brainstem, middle- and long-latency components. Hear Res 66:91–98. doi:10.1016/0378-5955(93)90263-Z
Melcher JR (1996) Cellular generators of the binaural difference potential in cat. Hear Res 95:144–160. doi:10.1016/0378-5955(96)00032-9
Moiseff A, Konishi M (1983) Binaural characteristics of units in the owl’s brainstem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci 3:2553–2562
Myoga MH, Lehnert S, Leibold C, Felmy F, Grothe B (2014) Glycinergic inhibition tunes coincidence detection in the auditory brainstem. Nat Commun 5:3790
Ohmori H (2014) Neuronal specializations for the processing of interaural difference cues in the chick. Front Neural Circuits 8:47. doi:10.3389/fncir.2014.00047
Overholt EM, Rubel EW, Hyson RL (1992) A circuit for coding interaural time differences in the chick brainstem. J Neurosci 12:1698–1708
Palanca-Castan N, Köppl C (2015) In vivo recordings from low-frequency nucleus laminaris in the barn owl. Brain Behav Evol 85:271–286. doi:10.1159/000433513
Pecka M, Brand A, Behrend O, Grothe B (2008) Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci 28:6914–6925. doi:10.1523/JNEUROSCI.1660-08.2008
Peña JL, Viete S, Albeck Y, Konishi M (1996) Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16:7046–7054
Pratt H, Polyakov A, Aharonson V, et al. (1998) Effects of localized pontine lesions on auditory brain-stem evoked potentials and binaural processing in humans. Electroencephalogr Clin Neurophysiol 108:511–520
Riedel H, Kollmeier B (2002a) Auditory brain stem responses evoked by lateralized clicks: is lateralization extracted in the human brain stem? Hear Res 163:12–26. doi:10.1016/S0378-5955(01)00362-8
Riedel H, Kollmeier B (2002b) Comparison of binaural auditory brainstem responses and the binaural difference potential evoked by chirps and clicks. Hear Res 169:85–96. doi:10.1016/S0378-5955(02)00342-8
Riedel H, Granzow M, Kollmeier B (2001) Single-sweep-based methods to improve the quality of auditory brain stem responses part II: averaging methods. Zeitschrift für Audiol 40:62–85
Roberts MT, Seeman SC, Golding NL (2013) A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron 78:923–935. doi:10.1016/j.neuron.2013.04.022
Takahashi TT, Konishi M (1988) Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol 274:212–238. doi:10.1002/cne.902740207
Tollin DJ (2003) The lateral superior olive: a functional role in sound source localization. Neuroscientist 9:127–143
Tollin DJ, Yin TCT (2005) Interaural phase and level difference sensitivity in low-frequency neurons in the lateral superior olive. J Neurosci 25:10648–10657. doi:10.1523/JNEUROSCI.1609-05.2005
Ungan P, Yagcioglu S (2002) Origin of the binaural interaction component in wave P4 of the short-latency auditory evoked potentials in the cat: evaluation of serial depth recordings from the brainstem. Hear Res 167:81–101. doi:10.1016/S0378-5955(02)00351-9
Ungan P, Yagcioglu S, Özmen B (1997) Interaural delay-dependent changes in the binaural difference potential in cat auditory brainstem response: implications about the origin of the binaural interaction component. Hear Res 106:66–82. doi:10.1016/S0378-5955(97)00003-8
van der Heijden M, Lorteije JAM, Plauška A, et al. (2013) Directional hearing by linear summation of binaural inputs at the medial superior olive. Neuron 78:936–948. doi:10.1016/j.neuron.2013.04.028
Wada S, Starr A (1989) Anatomical bases of binaural interaction in auditory brainstem responses from guinea pig
Wada SI, Starr A (1983a) Generation of auditory brain stem responses (ABRs). I. Effects of injection of a local anesthetic (procaine HCI) into the trapezoid body of Guinea pigs and cat. Electroencephalogr Clin Neurophysiol 56:326–339
Wada SI, Starr A (1983b) Generation of auditory brain stem responses (ABRs). III. Effects of lesions of the superior olive, lateral lemniscus and inferior colliculus on the ABR in Guinea pig. Electroencephalogr Clin Neurophysiol 56:352–366
Wada SI, Starr A (1983c) Generation of auditory brain stem responses (ABRs). II. Effects of surgical section of the trapezoid body on the ABR in Guinea pigs and cat. Electroencephalogr Clin Neurophysiol 56:340–351
Wernick JS, Starr A (1968) Binaural interaction in the superior olivary complex of the cat: an analysis of field potentials evoked by binaural-beat stimuli. J Neurophysiol 31:428–441
Young SR, Rubel EW (1983) Frequency-specific projections of individual neurons in chick brainstem auditory nuclei. J Neurosci 3:1373–1378
Zaaroor M, Starr A (1991) Auditory brain-stem evoked potentials in cat after kainic acid induced neuronal loss. I. Superior olivary complex. Electroencephalogr Clin Neurophysiol 80:422–435
Acknowledgments
Supported by the Deutsche Forschungsgemeinschaft (CRC/TRR31 “Active Hearing” and Cluster of Excellence “Hearing4All”). We thank Rainer Beutelmann for his technical assistance. Georg Klump provided invaluable input early in the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Palanca-Castan, N., Laumen, G., Reed, D. et al. The Binaural Interaction Component in Barn Owl (Tyto alba) Presents few Differences to Mammalian Data. JARO 17, 577–589 (2016). https://doi.org/10.1007/s10162-016-0583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0583-7