Abstract
Background
Hypertension is one of the major etiologies that cause chronic kidney disease (CKD) and can exacerbate kidney dysfunction. Zinc is an essential trace element playing a role in blood pressure regulation, and zinc deficiency, a common comorbidity in patients with CKD, can cause hypertension. However, the precise mechanism underlying zinc deficiency-induced hypertension is unknown. Sodium (Na+) retention due to inappropriate Na+ reabsorption in the renal tubule is the principal pathophysiology of hypertension. Therefore, this study aimed to investigate the association between zinc deficiency and salt sensitivity.
Methods
Adult mice were fed a zinc-adequate (ZnA) or zinc-deficient (ZnD) diet combined with/without high salt in drinking water (HS) for 4 weeks (n = 6 each). Changes in blood pressure, urinary sodium excretion, and the expressions of the proximal tubular Na+ transporter, Na+/H+ exchanger 3 (NHE3), which mostly contributes to filtered Na+ reabsorption and the downstream Na+–Cl− transporter (NCC) were analyzed.
Results
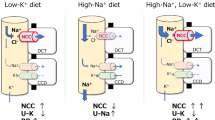
Urinary Na+ excretion significantly increased in ZnD mice, indicating that zinc deficiency causes natriuresis. NHE3 expressions were significantly suppressed, whereas NCC was upregulated in ZnD mice. Interestingly, the combination of high salt and ZnD diet (HS-ZnD) reversed the urinary Na+ loss. The NCC remained activated and NHE3 expressions paradoxically increased in HS-ZnD mice compared with those fed the combination of high salt and ZnA diet. In addition, blood pressure significantly increased only in HS-ZnD mice.
Conclusion
The combination of zinc deficiency and high salt causes hypertension. Zinc is associated with salt-sensitivity, potentially through NHE3 and NCC regulation.











Similar content being viewed by others
References
Kon S, Konta T, Ichikawa K, Asahi K, Yamagata K, Fujimoto S, Tsuruya K, Narita I, Kasahara M, Shibagaki Y, Iseki K, Moriyama T, Kondo M, Watanabe T. Association between renal function and cardiovascular and all-cause mortality in the community-based elderly population: results from the specific health check and guidance program in Japan. Clin Exp Nephrol. 2018;22:346–52.
Marreiros C, Viegas C, Simes D. Targeting a silent disease: vascular calcification in chronic kidney disease. Int J Mol Sci. 2022;23:16114.
Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 2000;57:1803–17.
Takata T, Isomoto H. Pleiotropic effects of sodium-glucose Cotransporter-2 inhibitors: renoprotective mechanisms beyond glycemic control. Int J Mol Sci. 2021;22:4374.
Milik A, Chapter HE. Management of progression and complications of CKD. Kidney Int Suppl. 2013;3:73–90.
Cortinovis M, Ruggenenti P, Remuzzi G. Progression, remission and regression of chronic renal diseases. Nephron. 2016;134:20–4.
Ohno S, Ishii A, Yanagita M, Yokoi H. Calcium channel blocker in patients with chronic kidney disease. Clin Exp Nephrol. 2022;26:207–15.
Bourque G, Hiremath S. Rethinking resistant hypertension. J Clin Med. 2022;11:1455.
Maaliki D, Itani MM, Itani HA. Pathophysiology and genetics of salt-sensitive hypertension. Front Physiol. 2022;13:1001434.
He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension. 2003;42:1093–9.
Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science. 1991;252:1813–6.
Trepiccione F, Zacchia M, Capasso G. The role of the kidney in salt-sensitive hypertension. Clin Exp Nephrol. 2021;16:68–72.
Nanamatsu A, Mori T, Ando F, Furusho T, Mandai S, Susa K, Sohara E, Rai T, Uchida S. Vasopressin induces urinary uromodulin secretion by activating PKA (protein kinase A). Hypertension. 2021;77:1953–63.
Takata T, Hamada S, Mae Y, Iyama T, Ogihara R, Seno M, Nakamura K, Takata M, Sugihara T, Isomoto H. Uromodulin regulates murine aquaporin-2 activity via thick ascending limb-collecting duct cross-talk during water deprivation. Int J Mol Sci. 2022;23:9410.
Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–6.
Bovée DM, Cuevas CA, Zietse R, Danser AHJ, Mirabito Colafella KM, Hoorn EJ. Salt-sensitive hypertension in chronic kidney disease: distal tubular mechanisms. Am J Physiol Ren Physiol. 2020;319:F729–45.
Takata T, Isomoto H. The versatile role of uromodulin in renal homeostasis and its relevance in chronic kidney disease. InternMed. 2024;63:17–23. https://doi.org/10.2169/internalmedicine.1342-22.
Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95:749–84.
Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71:3281–95.
Franceschini N, Chasman DI, Cooper-DeHoff RM, Arnett DK. Genetics, ancestry, and hypertension: implications for targeted antihypertensive therapies. Curr Hypertens Rep. 2014;16:461.
International Consortium for Blood Pressure Genome-Wide Association Studies, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9.
Kimura T, Kambe T. The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int J Mol Sci. 2016;17:336.
Hu R, McDonough AA, Layton AT. Functional implications of the sex differences in transporter abundance along the rat nephron: modeling and analysis. Am J Physiol Ren Physiol. 2019;317:F1462–74.
Gonzalez-Vicente A, Hong NJ, Yang N, Cabral PD, Berthiaume JM, Dominici FP, Garvin JL. Dietary fructose increases the sensitivity of proximal tubules to angiotensin II in rats fed high-salt diets. Nutrients. 2018;10:1244.
Olinger E, Lake J, Sheehan S, Schiano G, Takata T, Tokonami N, Debaix H, Consolato F, Rampoldi L, Korstanje R, Devuyst O. Hepsin-mediated processing of uromodulin is crucial for salt-sensitivity and thick ascending limb homeostasis. Sci Rep. 2019;9:12287.
Takata T, Munemura C, Fukui T, Fukuda S, Murawaki Y. Influence of olmesartan on sirtuin 1 mRNA expression in 5/6 nephrectomized spontaneously hypertensive rats. Yonago Acta Med. 2015;58:63–8.
Hosokawa K, Takata T, Sugihara T, Matono T, Koda M, Kanda T, Taniguchi S, Ida A, Mae Y, Yamamoto M, Iyama T, Fukuda S, Isomoto H. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipiddeposition in renal tubules. Int J Mol Sci. 2019;21:190.
Tokonami N, Takata T, Beyeler J, Ehrbar I, Yoshifuji A, Christensen EI, Loffing J, Devuyst O, Olinger EG. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int. 2018;94:701–15.
Lewis S, Chen L, Raghuram V, Khundmiri S, Chou CL, Yang CR, Knepper MA. “SLC-omics” of the kidney: solute transporters along the nephron. Am J Physiol Cell Physiol. 2021;321:C507-518.
Williams CR, Mistry M, Cheriyan AM, Williams JM, Naraine MK, Ellis CL, Mallick R, Mistry AC, Gooch JL, Ko B, Cai H, Hoover RS. Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. Am J Physiol Ren Physiol. 2019;316:F646–53.
Feng HL, Chen YH, Jeng SS. Effect of zinc supplementation on renal anemia in 5/6-nephrectomized rats and a comparison with treatment with recombinant human erythropoietin. Int J Mol Sci. 2019;20:4985.
Tubek S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res. 2007;117:39–51.
Tahir F, Majid Z, Qadar LT. Zinc deficiency: an independent risk factor for high blood pressure. J Pak Med Assoc. 2019;69:1578.
Ozyildirim S, Baltaci SB. Cardiovascular diseases and zinc. Biol Trace Elem Res. 2023;201:1615–26.
Ume AC, Wenegieme TY, Adams DN, Adesina SE, Williams CR. Zinc deficiency: a potential hidden driver of thedetrimental cycle of chronic kidney disease and hypertension. Kidney. 2023;360(4):398–404.
Bergomi M, Rovesti S, Vinceti M, Vivoli R, Caselgrandi E, Vivoli G. Zinc and copper status and blood pressure. J Trace Elem Med Biol. 1997;11:166–9.
Medeiros DM, Brown BJ. Blood pressure in young adults as influenced by copper and zinc intake. Biol Trace Elem Res. 1983;5:165–74.
Kim J. Dietary zinc intake is inversely associated with systolic blood pressure in young obese women. Nutr Res Pract. 2013;7:380–4.
Kunutsor SK, Laukkanen JA. Serum zinc concentrations and incident hypertension: new findings from a population-based cohort study. J Hypertens. 2016;34:1055–61.
Sato M, Yanagisawa H, Nojima Y, Tamura J, Wada O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: possible role of Cu/Zn-superoxide dismutase. Clin Exp Hypertens. 2002;24:355–70.
Dimitrova AA, Strashimirov D, Betova T, Russeva A, Alexandrova M. Zinc content in the diet affects the activity of Cu/ZnSOD, lipid peroxidation and lipid profile of spontaneously hypertensive rats. Acta Biol Hung. 2008;59:305–14.
Hatanaka M, Kaimori JY, Yamamoto S, Matsui I, Hamano T, Takabatake Y, Ecelbarger CM, Takahara S, Isaka Y, Rakugi H. Azilsartan improves salt sensitivity by modulating the proximal tubular Na+-H+ exchanger-3 in mice. PLoS One. 2016;11:
Author information
Authors and Affiliations
Contributions
Conceptualization: Marie Yamamoto, Tomoaki Takata Hinako Hanada, Sosuke Taniguchi, Shintaro Hamada, Yukari Mae, and Takuji Iyama; Formal analysis and investigation: Marie Yamamoto, Tomoaki Takata, Takuji Iyama, and Tsutomu Kanda; Writing—original draft preparation: Marie Yamamoto; Writing—review and editing: Tomoaki Takata; Supervision; Hajime Isomoto. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Approval was granted by the Ethics of Animal Experiments of the Tottori University (19-Y-31).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yamamoto, M., Takata, T., Hanada, H. et al. Zinc deficiency induces hypertension by paradoxically amplifying salt sensitivity under high salt intake in mice. Clin Exp Nephrol (2024). https://doi.org/10.1007/s10157-024-02478-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10157-024-02478-7