Abstract
Background
Febrile neutropenia represents a critical oncologic emergency, and its management is pivotal in cancer therapy. In several guidelines, the use of granulocyte colony-stimulating factor (G-CSF) in patients with chemotherapy-induced febrile neutropenia is not routinely recommended except in high-risk cases. The Japan Society of Clinical Oncology has updated its clinical practice guidelines for the use of G-CSF, incorporating a systematic review to address this clinical question.
Methods
The systematic review was conducted by performing a comprehensive literature search across PubMed, the Cochrane Library, and Ichushi-Web, focusing on publications from January 1990 to December 2019. Selected studies included randomized controlled trials (RCTs), non-RCTs, and cohort and case–control studies. Evaluated outcomes included overall survival, infection-related mortality, hospitalization duration, quality of life, and pain.
Results
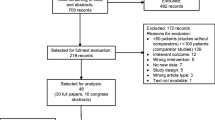
The initial search yielded 332 records. Following two rounds of screening, two records were selected for both qualitative and quantitative synthesis including meta-analysis. Regarding infection-related mortality, the event to case ratio was 5:134 (3.73%) in the G-CSF group versus 6:129 (4.65%) in the non-G-CSF group, resulting in a relative risk of 0.83 (95% confidence interval, 0.27–2.58; p = 0.54), which was not statistically significant. Only median values for hospitalization duration were available from the two RCTs, precluding a meta-analysis. For overall survival, quality of life, and pain, no suitable studies were found for analysis, rendering their assessment unfeasible.
Conclusion
A weak recommendation is made that G-CSF treatment not be administered to patients with febrile neutropenia during cancer chemotherapy. G-CSF treatment can be considered for patients at high risk.


Similar content being viewed by others
References
Weycker D, Li X, Edelsberg J et al (2015) Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract 11:47–54
Leon Rapoport B, Garcia-Morillo M, Font C et al (2023) A prospective, real-world, multinational study of febrile neutropenia (FN) occurrence in oncology patients receiving chemotherapy with intermediate risk of FN: a MASCC Neutropenia, Infection, and Myelosuppression Study Group initiative. Support Care Cancer 31:628
Klastersky J, de Naurois J, Rolston K et al (2016) Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol 27:v111–v118
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
NCCN Clinical Practice Guidelines in Oncology. Hematopoietic growth factors version 3. 2024. Accessed 7 Mar 2024
Medical Information Network Distribution System: MINDS guideline center. http://minds.jcqhc.or.jp/n/st.php. Accessed 7 Mar 2024
Andrews J, Guyatt G, Oxman AD et al (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725
Andrews JC, Schünemann HJ, Oxman AD et al (2013) GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation’s direction and strength. J Clin Epidemiol 66:726–735
García-Carbonero R, Mayordomo JI, Tornamira MV et al (2001) Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. J Natl Cancer Inst 93:31–38
Er O, Coskun HS, Altinbas M et al (2004) Meropenem +/− granulocyte colony stimulating factor in the treatment of febrile neutropenic patients with cancer: prospective randomized study. J Chemother 16:288–292
Mhaskar R, Clark OAC, Lyman G et al (2014) Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev 2014:CD003039
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Acknowledgements
The authors would like to thank Ms. Keiko Kato for conducting the initial literature search and Professor Masahiro Yoshida of Minds Tokyo Grading of Recommendations, Assessment, Development, and Evaluation Center for his advice regarding methodology. We would also like to thank Ms. Natsuki Fukuda for providing assistance to the study to formulate these guidelines.
Author information
Authors and Affiliations
Contributions
This study forms part of the updating of the Clinical Practice Guidelines for the Use of G-CSF, under the chairmanship of T.T. All authors contributed to the conceptualization and design of this study. The formal analysis was conducted by Y.O., K.N., and E.B.; K.T. and M.I. undertook the review of the analyzed data. The initial draft of the manuscript was composed by K.T., with E.B. providing supervision. M.I., Y.O., and K.N. reviewed and edited the draft. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kenji Tsuchihashi has received honoraria from Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co., Taiho Pharmaceutical Co. Ltd., and Novartis Pharmaceutical. K.K. Yukinori Ozaki has received honoraria from Daiichi Sankyo Co. Ltd., Pfizer Japan Inc., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., and Kyowa Kirin Co. Ltd. Eiki Ichihara has received honoraria from Eli Lilly Japan K.K. and research funding from MSD K.K., Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., and Takeda Pharmaceutical Co. Ltd. Yuji Miura has received honoraria from Ono Pharmaceutical Co. Ltd., MSD K.K., Takeda Pharmaceutical Co. Ltd., Eisai Co. Ltd., and Bristol Myers Squibb Co. Ltd., and research funding from MSD K.K. and Ono Pharmaceutical Co. Ltd. Shingo Yano has received research funding from Otsuka Pharmaceutical Co. Ltd. Dai Maruyama has received honoraria from Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., Nippon Shinyaku Co. Ltd., Eisai Co. Ltd., Mundipharma, Kyowa Kirin Co. Ltd., Chugai Pharmaceutical Co. Ltd., Zenyaku Holdings Co. Ltd., MSD K.K., SymBio Pharmaceutical Ltd., Sanofi K.K., AbbVie Inc., Takeda Pharmaceutical Co. Ltd., AstraZeneca K.K., Bristol Myers Squibb Co. Ltd., and Genmab K.K., and research funding from Amgen K.K., Astellas Pharma Inc., Biopharma Co. Ltd., Novartis Pharmaceutical K.K., Kyowa Kirin Co. Ltd., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Sanofi K.K., Bristol Myers Squibb Co. Ltd., AbbVie Inc., Eisai Co. Ltd., MSD K.K., Taiho Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., and Genmab K.K. Tetsuhiro Yoshinami has received honoraria from Kyowa Kirin Co. Ltd., Pfizer Japan Inc., Chugai Pharmaceutical Co., Eli Lilly Japan K.K., MSD K.K., AstraZeneca K.K., and Eisai Co. Ltd. Takashi Motohashi has received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., and Myriad Genetics G.K. Toshio Kubo has received honoraria from Chugai Pharmaceutical Co. Takahiro Kimura has received honoraria from Sanofi K.K. Shinji Nakao has received honoraria from Kyowa Kirin Co., Ltd. Atsushi Sato has received honoraria from Chugai Pharmaceutical Co. and Taiho Pharmaceutical Co. Ltd., and a scholarship donation from Chugai Pharmaceutical Co. and Taiho Pharmaceutical Co. Ltd. Toshimi Takano has received honoraria from Daiichi Sankyo Co. Ltd., Chugai Pharmaceutical Co., and Eli Lilly Japan K.K. Eishi Baba has received honoraria from Chugai Pharmaceutical Co. Ltd. and Daiichi Sankyo Co.Ltd., and research funding from Taiho Pharmaceutical Co. Ltd. and Chugai Pharmaceutical Co. Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tsuchihashi, K., Ito, M., Okumura, Y. et al. Therapeutic use of granulocyte colony-stimulating factor (G-CSF) in patients with febrile neutropenia: a comprehensive systematic review for clinical practice guidelines for the use of G-CSF 2022 from the Japan Society of Clinical Oncology. Int J Clin Oncol 29, 700–705 (2024). https://doi.org/10.1007/s10147-024-02541-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02541-z