Abstract
Background
Neoadjuvant chemotherapy is a common therapeutic procedure for patients with pancreatic cancer. This study aimed to investigate the association between the total psoas area (TPA) and prognosis in patients undergoing neoadjuvant chemotherapy for resectable or borderline resectable pancreatic cancer.
Study design
This retrospective study included patients who underwent neoadjuvant chemotherapy for pancreatic cancer. TPA was measured at the level of the L3 vertebra using computed tomography. The patients were divided into low-TPA and normal-TPA groups. These dichotomizations were separately performed in patients with resectable and those with borderline resectable pancreatic cancer.
Results
In total, 44 patients had resectable pancreatic cancer and 71 patients had borderline resectable pancreatic cancer. Overall survival among patients with resectable pancreatic cancer did not differ between the normal- and low-TPA groups (median, 19.8 vs. 21.8 months, p = 0.447), whereas among patients with borderline resectable pancreatic cancer, the low-TPA group had shorter overall survival than the normal-TPA group (median, 21.8 vs. 32.9 months, p = 0.006). Among patients with borderline resectable pancreatic cancer, the low-TPA group was predictive of poor overall survival (adjusted hazard ratio, 2.57, p = 0.037).
Conclusion
Low TPA is a risk factor of poor survival in patients undergoing neoadjuvant chemotherapy for borderline resectable pancreatic cancer. TPA evaluation could potentially suggest the treatment strategy in this disease.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
References
Kleeff J, Korc M, Apte M et al (2016) Pancreatic cancer. Nat Rev Dis Primers 2:16022
Griffin O, Conlon KC (2018) Sarcopenia-a new frontier in the management care of patients with borderline resectable pancreatic cancer. JAMA Surg 153(9):816
Motoi F, Kosuge T, Ueno H et al (2019) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 49(2):190–194
van Dam JL, Janssen QP, Besselink MG et al (2022) Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 160:140–149
Bundred J, Kamarajah SK, Roberts KJ (2019) Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 21(12):1603–1612
Pierobon ES, Moletta L, Zampieri S et al (2021) The prognostic value of low muscle mass in pancreatic cancer patients: a systematic review and meta-analysis. J Clin Med 10(14):3033
Griffin OM, Duggan SN, Ryan R et al (2019) Characterising the impact of body composition change during neoadjuvant chemotherapy for pancreatic cancer. Pancreatology 19(6):850–857
Jin K, Tang Y, Wang A et al (2022) Body composition and response and outcome of neoadjuvant treatment for pancreatic cancer. Nutr Cancer 74(1):100–109
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85(9):1001–1005
McMillan DC, Crozier JE, Canna K et al (2007) Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 22(8):881–886
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Yamaguchi J, Yokoyama Y, Fujii T et al (2022) Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann Surg 275(6):1043–1049
da Rocha IMG, Marcadenti A, de Medeiros GOC et al (2019) Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle 10(2):445–454
Evans DB, Rich TA, Byrd DR et al (1992) Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127(11):1335–1339
Clavien PA, Strasberg SM (2009) Severity grading of surgical complications. Ann Surg 250(2):197–198
Peng P, Hyder O, Firoozmand A et al (2012) Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 16(8):1478–1486
Delitto D, Judge SM, George TJ et al (2017) A clinically applicable muscular index predicts long-term survival in resectable pancreatic cancer. Surgery 161(4):930–938
Otsuji H, Yokoyama Y, Ebata T et al (2015) Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg 39(6):1494–1500
Otsuji H, Yokoyama Y, Ebata TI et al (2017) Surgery-related muscle loss and its association with postoperative complications after major hepatectomy with extrahepatic bile duct resection. World J Surg 41(2):498–507
Mogal H, Vermilion SA, Dodson R et al (2017) Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 24(6):1714–1721
Mintziras I, Miligkos M, Wächter S et al (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg 59:19–26
Cooper AB, Slack R, Fogelman D et al (2015) Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 22(7):2416–2423
Nakajima H, Yokoyama Y, Inoue T et al (2019) Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol 26(1):264–272
Duggal NA, Niemiro G, Harridge SDR et al (2019) Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol 19(9):563–572
Narsale A, Moya R, Ma J et al (2019) Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. J Cachexia Sarcopenia Muscle 10(4):827–843
Looijaard SMLM, Te Lintel Hekkert ML, Wüst RCI et al (2021) Pathophysiological mechanisms explaining poor clinical outcome of older cancer patients with low skeletal muscle mass. Acta Physiol (Oxf) 231(1):e13516
Yokoyama Y, Nagino M, Ebata T (2021) Importance of “muscle” and “intestine” training before major HPB surgery: a review. J Hepatobiliary Pancreat Sci 28(7):545–555
Acknowledgements
We sincerely thank all the patients, collaborating physicians, and other medical staff for their important contributions to this study.
Funding
The authors affirm that they have no financial or personal affiliations (including research funding) or other involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript.
Author information
Authors and Affiliations
Contributions
HN: conceptualization, data curation, writing. JY: data curation. HT: data curation. MH: data curation. YK: data curation. YN: data curation. NW: data curation. SO: data curation. TM: data curation. YY: conceptualization, data curation, writing, validation. TE: conceptualization, validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2023_2321_MOESM1_ESM.pptx
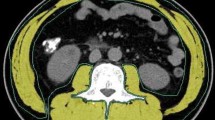
Supplementary file1 Supplementary Fig. 1 Abdominal computed tomography image at the level of the third lumbar vertebra. The right and left psoas muscle areas, which comprise the total psoas muscle area (TPA), were traced (dotted line). Normalized TPA = measured TPA [mm2]/height [m]2. Supplementary Fig. 2 Kaplan–Meier curves for recurrence free survival in patients with (A) resectable pancreatic cancer and (B) borderline resectable pancreatic cancer. (PPTX 203 kb)
About this article
Cite this article
Nakajima, H., Yamaguchi, J., Takami, H. et al. Impact of skeletal muscle mass on the prognosis of patients undergoing neoadjuvant chemotherapy for resectable or borderline resectable pancreatic cancer. Int J Clin Oncol 28, 688–697 (2023). https://doi.org/10.1007/s10147-023-02321-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02321-1