Abstract
Background
Lymphatic metastasis is a major route of metastasis in distal cholangiocarcinoma (DCC). The present study aimed to elucidate the pattern of lymph node (LN) metastasis and the effectiveness of LN dissection and postoperative adjuvant chemotherapy in patients with DCC.
Methods
Patients who underwent surgical resection with curative intent for DCC were enrolled. The nomenclature of the LN stations was defined according to the Japanese Society of Hepato-Biliary-Pancreatic Surgery guidelines. Effectiveness of LN dissection of each station was calculated using frequency of LN metastasis to the station and 5-year survival rate of patients with LN metastasis to that station.
Results
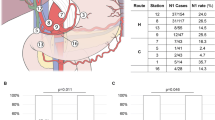
Of the 105 patients included in the study, 46 (43.8%) had LN metastasis, and 43 (41.0%) underwent postoperative adjuvant therapy. LN metastasis, serum carbohydrate antigen (CA) 19–9 level > 37 U/mL, and positive bile duct margin were independent risk factors for shorter overall survival (OS). The most common metastatic LN station at surgery was No. 13 (32.7%), followed by No. 12 (19.2%), No. 17 (9.6%), and No. 8 (6.6%). There was no effectiveness of LN dissection of the station No. 8, 14, and 16. Adjuvant chemotherapy was significantly associated with longer OS in patients with LN metastasis but not in those with positive ductal margins or serum CA 19–9 level > 37 U/mL.
Conclusions
Postoperative adjuvant chemotherapy was associated with a better prognosis in patients with DCC and LN metastasis. However, a more effective therapeutic strategy is required to improve the prognosis of patients with other negative prognostic factors.



Similar content being viewed by others
References
Razumilava N, Gores GJ (2014) Cholangiocarcinoma. Lancet 383:2168–2179
Al Mahjoub A, Bouvier V, Menahem B et al (2019) Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. Eur J Gastroenterol Hepatol 31:678–684
Ercolani G, Dazzi A, Giovinazzo F et al (2015) Intrahepatic, peri-hilar and distal cholangiocarcinoma: three different locations of the same tumor or three different tumors? Eur J Surg Oncol 41:1162–1169
Andrianello S, Paiella S, Allegrini V et al (2015) Pancreaticoduodenectomy for distal cholangiocarcinoma: surgical results, prognostic factors, and long-term follow-up. Langenbecks Arch Surg 400:623–628
Murakami Y, Uemura K, Hayashidani Y et al (2015) Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg 102:399–406
Li X, Lin H, Sun Y et al (2019) Prognostic significance of the lymph node ratio in surgical patients with distal cholangiocarcinoma. J Surg Res 236:2–11
Chung MJ, Lee KJ, Bang S et al (2011) Preoperative serum CA 19–9 level as a predictive factor for recurrence after curative resection in biliary tract cancer. Ann Surg Oncol 18:1651–1656
Murakami Y, Uemura K, Sudo T et al (2011) Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 18:651–658
Kurahara H, Maemura K, Mataki Y et al (2017) Relationship between the surgical margin status, prognosis, and recurrence in extrahepatic bile duct cancer patients. Langenbecks Arch Surg 402:87–93
Chun YS, Pawlik TM, Vauthey JN (2018) 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol 25:845–847. https://doi.org/10.1245/s10434-017-6025-x
Hester C, Nassour I, Adams-Huet B et al (2018) Improved survival in surgically resected distal cholangiocarcinoma treated with adjuvant therapy: a propensity score matched analysis. J Gastrointest Surg 22:2080–2087
Bergeat D, Turrini O, Courtin-Tanguy L et al (2018) Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: a propensity score analysis from a French multicentric cohort. Langenbecks Arch Surg 403:701–709
Messina C, Merz V, Frisinghelli M et al (2019) Adjuvant chemotherapy in resected bile duct cancer: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol 143:124–129
Sasako M, McCulloch P, Kinoshita T et al (1995) New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 82:346–351
Imamura T, Yamamoto Y, Sugiura T et al (2021) Reconsidering the optimal regional lymph node station according to tumor location for pancreatic cancer. Ann Surg Oncol 28:1602–1611
Miyazaki M, Ohtsuka M, Miyakawa S et al (2015) Classification of biliary tract cancers established by the Japanese society of hepato-biliary-pancreatic surgery 3(rd) English edition. J Hepatobiliary Pancreat Sci 22:181–196
Neoptolemos JP, Moore MJ, Cox TF et al (2012) Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 308:147–156
Ebata T, Hirano S, Konishi M et al (2018) Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 105:192–202
Edeline J, Benabdelghani M, Bertaut A et al (2019) Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase iii study. J Clin Oncol 37:658–667
Primrose JN, Fox RP, Palmer DH et al (2019) Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 20:663–673
Furuse J, Okusaka T, Boku N et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Okusaka T, Ishii H, Funakoshi A et al (2006) Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 57:647–653
Kobayashi S, Nagano H, Tomokuni A et al (2019) A prospective, randomized phase ii study of adjuvant gemcitabine versus S-1 after major hepatectomy for biliary tract cancer (KHBO 1208): kansai hepato-biliary oncology group. Ann Surg 270:230–237
Itano O, Takemura Y, Kishida N et al (2020) A prospective feasibility study of one-year administration of adjuvant S-1 therapy for resected biliary tract cancer in a multi-institutional trial (Tokyo Study Group for Biliary Cancer: TOSBIC01). BMC Cancer 20:688. https://doi.org/10.1186/s12885-020-07185-6
Yadav S, Xie H, Bin-Riaz I et al (2019) Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: a propensity score matched analysis. Eur J Surg Oncol 45:1432–1438
Acknowledgements
This study was funded by a Grants-in-Aid for Scientific Research (20K21633) from the Japan Society for the Promotion of Science, which is under the direction of the Ministry of Health, Labor, and Welfare, Japan.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Acquisition of data was performed by YK, SM, KS, TA, and AN. Analysis and interpretation of data were performed by TI, YM, YF, and MH. The first draft of the manuscript was written by HK, and all authors commented on the previous version of the manuscript. Critical revision of the manuscript was performed by HK and TO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and/or animals participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kurahara, H., Mataki, Y., Idichi, T. et al. Spread of lymph node metastasis and adjuvant therapy for distal cholangiocarcinoma. Int J Clin Oncol 27, 1212–1221 (2022). https://doi.org/10.1007/s10147-022-02175-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02175-z