Abstract
Purpose
Gastrointestinal cancer is frequently associated with malignant ascites, resulting in poor prognosis. While cell-free and concentrated ascites reinfusion therapy (CART) improves ascites-related symptoms, its clinical impact in combination with systemic chemotherapy is unclear. The purpose of this study was to evaluate the safety and efficacy of CART in gastrointestinal cancer patients with massive ascites treated with chemotherapy.
Methods
We retrospectively reviewed the medical records of gastrointestinal cancer patients with massive ascites who received CART and chemotherapy at our hospital between July 2015 and September 2017.
Results
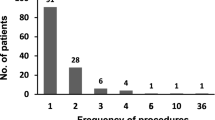
A total of 30 patients received CART and chemotherapy: gastric cancer (n = 21) and colorectal cancer (n = 9). The initial CART improved performance status in 20% of the patients, and the mean serum albumin and creatinine was significantly improved. Median time to treatment failure and overall survival of chemotherapy following CART were 2.1 and 3.5 months in gastric cancer patients and 5.8 and 5.8 months in colorectal cancer patients, respectively. The frequency of paracentesis was decreased after introduction of CART followed by chemotherapy in 83% of gastric cancer and in all colorectal cancer patients who had received paracentesis before the initial CART. There were no grade 3/4 adverse events during the CART procedure. Grade 3/4 hematotoxic and non-hematotoxic adverse events of chemotherapy following CART were 30% and less than 10%, respectively.
Conclusions
The combination of CART followed by chemotherapy is safe and could be a treatment option for gastrointestinal cancer patients with massive ascites.



Similar content being viewed by others
References
Runyon BA (1994) Care of patients with ascites. N Engl J Med 330:337–342
Runyon BA, Hoefs J, Morgan TR et al (1988) Ascitic fluid analysis in malignancy-related ascites. Hepatology. 8:1104–1109
Saif MW, Siddiqui IAP, Sohail MA (2009) Management of ascites due to gastrointestinal malignancy. Ann Saudi Med 29:369–377
Garisson RN, Kaelin LD, Galloway RH et al (1986) Malignant ascites. Clinical and experimental observations. Ann Surg 203:644–651
Ito T, Hanafusa N (2017) CART: cell-free and concentrated ascites reinfusion therapy against malignancy-related ascites. Transfus Apher Sci 56:703–707
Britton RC, Nakamoto S et al (1960) Intravenous infusion of dialyzed, autogenous, ascetic fluid in the management of cirrhotic ascites: a preliminary report of favorable results in six patients. Cleve Clin Q 27:82–87
Inoue N, Yamazaki Z, Oda T, Sugiura M, Wada T (1977) Treatment of intractable ascites by continuous reinfusion of sterilized, cell-free and concentrated ascetic fluid. Trans Am Soc Artif Intern Organs 23:699–702
Ito T, Hanafusa N, Fukui M et al (2014) Single center experience of cell-free and concentrated ascites re-infusion therapy in malignancy related ascites. Ther Apher Dial 18:87–92
Hanafusa N, Isoai A, Ishihara T et al (2017) Safety and efficacy od cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: post-marketing surveillance results. PLoS One:e0177303
Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, Yamada Y, Shimada Y (2011) Ssystematic chemotherapy for peritoneal deisseminated gastric cancer with inadequate oral intake: a retrospective study. Int J Clin Oncol 16:57–62
Japanese Gastric Cancer Association (2014) Japanese cancer treatment guidelines
Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer
Wang L, Okubo T, Shinsaki M et al (2015) Efficacy and safety of cell-free and concentrated ascites reinfusion therapy (CART) in gynecologic cancer patients with a large volume of ascites. J Obstet Gynecol Res 41:1614–1620
Kamimura H, Yamagiwa S, Takamura M et al (2014) Efficacy of cell-free and concentrated ascites reinfusion therapy for malignant ascites. JSM Gastroenterol Hepatol 2:1027
Ito T, Hanafusa N, Iwase S, Noiri E, Nangaku M, Nakagawa K, Miyagawa K (2015) Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int J Clin Oncol 20:623–628
Andus T, Gross V, Holstage A et al (1992) Evidence for the production of high amount of interleukin-6 in the peritoneal cavity of patients with ascites. J Hepotol 15:378–381
Ueda T, Maehara M, Takahashi Y et al (2012) Clinical significance of cell-free and concentrated ascites re-infusion therapy for advanced and recurrent gynecological cancer. Anticancer Res 32:2353–2358
Yamaguchi H, Kitayama J, Emoto S, Ishigami H, Ito T, Hanafusa N, Watanabe T (2015) Cell-free and concentrated ascites reinfusion therapy (CART) for malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S-1. Eur J Surg Oncol 41:875–880
Nakajima TE, Yamaguchi K, Boku N et al (2020) Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin pluspaclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer. https://doi.org/10.1007/s10120-020-01043-x
Sherer DM, Eliakim R, Abulafia O (2000) The role of angiogenesis in the accumulation of peritoneal fluid in benign conditions and the development of malignant ascites in the female. Gynecol Obstet Investig 50:217–224
Takashima A, Shitara K, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Fujii H, Muro K, Esaki T, Choda Y, Takano T, Chin K, Sato A, Goto M, Fukushima N, Hara T, Machida N, Ohta M, Boku N, Shimura M, Morita S, Koizumi W (2018) Peritoneal metastasis as a predictive factor for nab-paclitaxel in patients with pretreated advanced gastric cancer: an exploratory analysis of the phase III ABSOLUTE trial. Gastric Cancer 22:155–163. https://doi.org/10.1007/s10120-018-0838-6
Imai Y, Hasegawa K, Matsushita H et al (2018) Expression of multiple immune check point molecules on T cells in malignant ascites from epithelial ovarian carcinoma. Oncol Lett 15:6457–6468
Nakano M, Ito M, Tanaka R, Yamaguchi K, Ariyama H, Mitsugi K, Yoshihiro T, Ohmura H, Tsuruta N, Hanamura F, Sagara K, Okumura Y, Nio K, Tsuchihashi K, Arita S, Kusaba H, Akashi K, Baba E (2018) PD-1+ TIM-3+ T cells in malignant ascites predict prognosis of gastrointestinal cancer. Cancer Sci 109:2986–2992
Acknowledgments
We would like to express our sincere thanks to all patients and investigators.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was reviewed and approved by the Institutional Review Board in National Cancer Center Hospital in Japan, number 2017-229. The Institutional Review Board decided that individual written or verbal consent could be waived because of the retrospective and observational nature of the current study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagata, Y., Kato, K., Miyamoto, T. et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support Care Cancer 28, 5861–5869 (2020). https://doi.org/10.1007/s00520-020-05401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05401-4