Abstract
Background
Peritoneal cytology positive for carcinoma cells (CY+) is an independent poor prognostic factor in gastric cancer, and patients with CY+ are diagnosed with stage IV disease. However, there is no standard treatment strategy for CY+ gastric cancer, whereas combination chemotherapy with fluoropyrimidine and platinum has been established as the standard treatment for unresectable advanced gastric cancer or after R2 resection. Herein, we assessed whether adding cisplatin to S-1 (SP) could improve the outcome of CY+ gastric cancer patients, as compared to S-1 monotherapy.
Methods
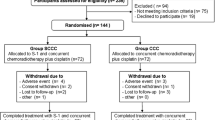
This retrospective study was conducted at a single Japanese institute between June 2005 and March 2014. Patients diagnosed with CY+ advanced gastric cancer and treated with S-1-based therapy were enrolled. Patients with incurable factors other than CY+ were excluded.
Results
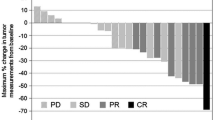
Forty-four patients were enrolled; 25 and 19 were administered S-1 and SP, respectively. The 2-year survival rates were 52.0% [95% confidence interval (CI), 31.2–69.2%] and 52.6% (28.7–71.9%) in the S-1 and SP groups, respectively. The median overall survival (OS) and progression-free survival (PFS) were 28.2 and 15.6 months in the S-1 group and 24.0 and 18.8 months in the SP group, respectively; they were not significantly different. The relative dose intensities were 0.79 (S-1) in the S-1 group and 0.69 (S-1)/0.70 (cisplatin) in the SP group.
Conclusion
Adding cisplatin to long-term S-1 monotherapy did not significantly improve the outcome of CY+ advanced gastric cancer patients.



Similar content being viewed by others
References
World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed 26 April 2016
Nakajima T, Harashima S, Hirata M et al (1978) Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol 22:225–229
Ikeguchi M, Oka A, Tsujitani S et al (1994) Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res 14:2131–2134
Bonenkamp JJ, Songun I, Hermans J et al (1996) Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 83:672–674
Kodera Y, Yamamura Y, Shimizu Y et al (1999) Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol 72:60–65
Bando E, Yonemura Y, Takeshita Y et al (1999) Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg 178:256–262
Burke EC, Karpeh MS Jr, Conlon KC et al (1998) Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol 5:411–415
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma, 3rd English edition. Gastric Cancer 1:10–24
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17:3077–3079
Kodera Y, Ito S, Mochizuki Y et al (2009) A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol 35:1158–1163
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Yamada Y, Higuchi K, Nishikawa K et al (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 26:141–148
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Cutsem Van, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Ajani JA, Rodriguez W, Bodoky G et al (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma, 3rd English edition. Gastric Cancer 14:101–112
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Noh SH, Park SR, Yang HK et al (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15:1389–1396
Takahari D, Hamaguchi T, Yoshimura K et al (2011) Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol 67:1423–1428
Takahari D, Hamaguchi T, Yoshimura K et al (2014) Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer 17:383–386
Cabalag CS, Chan ST, Kaneko Y et al (2015) A systematic review and meta-analysis of gastric cancer treatment in patients with positive peritoneal cytology. Gastric Cancer 18:11–22
Bando H, Yamada Y, Tanabe S et al (2016) Efficacy and safety of S-1 and cisplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer 19:919–926
Fujitani K, Yang HK, Mizusawa J et al (2016) Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 17:309–318
Nakagawa S, Nashimoto A, Yabusaki H (2007) Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer 10:29–34
Lorenzen S, Panzram B, Rosenberg R et al (2010) Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol 17:2733–2739
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Fuchs CS, Tomasek J, Yong CJ et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39
Yonemura Y, Endou Y, Bando E et al (2006) The usefulness of oral TS-1 treatment for potentially curable gastric cancer patients with intraperitoneal free cancer cells. Cancer Treat 4:135–142
Acknowledgments
We thank all staff who managed the study patients at the ambulatory treatment center and on the wards. We also thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.S. received lecture fees from Eli Lilly Japan K.K, Merck Serono, Takeda, Chugai, Taiho Pharmaceutical, Bayer Yakuhin, Ltd, and Yakult Hosha Co., Ltd and had acceptance of researchers from Toppan Printing Co., Ltd. T.I. received lecture fee from Eli Lilly Japan K.K and research grants from Clinico Co., Ltd. Y.K. received lecture fees from Eli Lilly Japan K.K, Merck Serono, Takeda, Chugai, Taiho Pharmaceutical and Yakult Hosha Co., Ltd, and received research grants from Taiho Pharmaceutical and Yakult Honsha Co., Ltd. The other authors declare that they have no conflict of interest.
About this article
Cite this article
Nakayama, I., Chin, K., Matsushima, T. et al. Retrospective comparison of S-1 plus cisplatin versus S-1 monotherapy for the treatment of advanced gastric cancer patients with positive peritoneal cytology but without gross peritoneal metastasis. Int J Clin Oncol 22, 1060–1068 (2017). https://doi.org/10.1007/s10147-017-1164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1164-4