Abstract
Mini abstract
In older patients with cT1N0M0 gastric cancer in the middle third of the stomach, LPPG has advantages over LDGB1 in maintaining skeletal muscle mass.
Background
Laparoscopic pylorus-preserving gastrectomy (LPPG) for early gastric cancer in the middle third of the stomach is expected to be an alternative procedure to laparoscopic distal gastrectomy (LDG). However, whether LPPG is safe and more useful than LDG in older patients is unclear because of their comorbidities and organ dysfunctions.
Methods
We retrospectively analyzed the data of consecutive patients aged 75 or over who underwent LDG with Billroth I reconstruction (LDGB1) or LPPG for cT1N0M0 gastric cancer in the middle third of the stomach between 2005 and 2019. After propensity score matching was used to improve the comparability between the LDGB1 and LPPG groups, we compared surgical and postoperative nutritional outcomes, including the postoperative trends of bodyweight (%BW) and skeletal muscle index (%SMI).
Results
A total of 132 patients who underwent LDGB1 (n = 88) and LPPG (n = 44) were collected for this study. No significant difference in postoperative complications was observed. The total protein levels after LPPG were significantly higher than those after LDGB1 for 4 postoperative years. Both %BW and %SMI after LPPG were significantly maintained compared with those after LDGB1 during the first year after surgery. For the subsequent years, %BW after LPPG became similar to that after LDGB1, while %SMI after LPPG was significantly larger than LDGB1 continuously.
Conclusions
LPPG has a great advantage in maintaining the postoperative skeletal muscle mass as well as the nutritional parameters of older patients. LPPG is expected to be an alternative to LDG in older patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most prevalent tumors and the third leading cause of deadly neoplasms worldwide [1]. The recent improvements in diagnostic techniques have enabled the early detection of gastric cancer and prolonged overall survival after gastrectomy [2], and hence treatments for early gastric cancer have shifted to less-invasive and more function-preserving procedures to improve postoperative quality of life (QOL).
Pylorus-preserving gastrectomy (PPG) is a function-preserving gastrectomy for early gastric cancer in the middle third of the stomach and has been recently performed laparoscopically (LPPG) as a less invasive surgery. PPG is expected to be an alternative procedure to conventional distal gastrectomy (DG) because it is predicted to have a lower incidence of bile reflux and post-gastrectomy syndromes such as dumping syndrome compared with DG with Billroth I reconstruction (DGB1) [3]. Several studies have revealed the potential advantages of PPG [4, 5].
Conversely, PPG has several limitations. First, PPG has specific complications such as gastric stasis or delayed gastric emptying, which sometimes causes nausea, vomiting, and postprandial fullness. Second, PPG is a more complicated surgical technique than conventional DG [6]. Third, solid evidence regarding the advantages of PPG compared with DG has not been established because we have limited data from retrospective or single-institutional studies. Thus, because of these problems of PPG, some surgeons feel that older patients should be excluded from the indication for PPG. Older patients may be more likely to suffer from delayed gastric emptying [7] and the complicated procedures of PPG may be a burden for older patients. Furthermore, the advantages with which PPG provides older patients during their short life spans are unclear.
Japan is the world’s most aging country. The proportion of older patients with early gastric cancer is increasing, and patients over 75 years of age account for almost half of all patients [8]. Currently, older patients in good health have a long life expectancy even after gastrectomy and their need for improved postoperative QOL arises. Surgeons must respond to the trend of aging patients, but the optimal strategy is still controversial because the safety and usefulness of PPG exclusively for older patients have not been reported. Therefore, whether PPG is also suitable for older patients needs to be clarified.
In this study, we compared the clinical outcomes after LPPG with conventional laparoscopic DGB1 (LDGB1) to determine whether LPPG is an advantageous procedure in older patients. The results obtained from this study will help surgeons to decide whether to select LPPG for older patients with early gastric cancer in the middle third of the stomach.
Methods
Patient selection
The data of patients aged 75 years and over who underwent LDG or LPPG for cT1N0M0 gastric cancer in the middle third of the stomach at the Cancer Institute Hospital, Tokyo, Japan, between January 2005 and December 2019, were retrospectively collected from our prospectively developed database. In addition to tumor status, patients were selected according to the following criteria: patients who had no hiatus hernia or esophageal reflux preoperatively; patients who underwent LDG or LPPG; and patients who underwent additional gastrectomy after endoscopic submucosal dissection. The exclusion criterion was synchronous cancer. In the LDG group, patients who underwent LDGB1 were limitedly collected in this study to avoid the confounding effect of food passage through the duodenum. Clinical and pathological stages were classified according to the 14th edition of the Japanese Classification of Gastric Carcinoma (3rd English edition) [9]. In pathological diagnosis, tubular differentiated and papillary adenocarcinoma were classified into differentiated type, and poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma were classified into undifferentiated type. This study was approved by the Institutional Review Board of the Cancer Institute Hospital (2021-GB-023).
Surgical procedure
LDGB1
Each patient in the LDG group satisfied the same criteria as LPPG but underwent the LDG procedure mainly because of old age. At our institution, several surgeons had not previously performed LPPG for older patients even though they had adequate lesions and conditions. In LDG, D1 + lymphadenectomy was performed, which involved the dissection of lymph node stations no. 1, 3a, 3b, 4sb, 4d, 5, 6, 7, 8a, and 9. The proximal side of the stomach was usually transected on the level of the meeting point of the bilateral gastroepiploic vessels in the greater curvature. After dissection and transection of the stomach extracorporeally or intracorporeally using a linear stapler, a Billroth I-type gastroduodenostomy was created via a small laparotomy to use a circular stapler or intracorporeally for the delta-shape anastomosis using a linear stapler.
LPPG
Recently, we have applied LPPG to older patients according to the surgeons’ preference when they did not have factors associated with contraindications for LPPG, such as preoperative hiatal hernia and reflux esophagitis. The detailed procedures of LPPG were described in our previous report [10]. Modified D1 + lymphadenectomy (dissection of lymph node stations no. 1, 3a, 3b, 4sb, 4d, 6, 7, 8a, and 9) was performed at our hospital. Consistent with our previous study [6], in addition to the preservation of the infrapyloric artery, the infrapyloric vein was preserved to keep venous drainage in the pyloric cuff. Because the right gastroepiploic artery and vein were transected after the bifurcation of infrapyloric vessels, the lymph node dissection of station no. 6 was partially restricted. The lymph node dissection of station no. 5 was not performed, but the sampling of station no. 5 lymph nodes was added when their metastasis was suspected. The hepatic and pyloric branches of the vagus nerve were preserved to maintain the pyloric function. Marking clips were preoperatively placed on the pathologically intact mucosa proximal and distal to the tumor edge. The stomach was transected with an endoscopic linear stapler, confirming these marking clips by touching them via a small laparotomy or under the guidance of intraoperative endoscopy. The proximal side of the transection line was consistent with the level of the meeting point of the bilateral gastroepiploic vessels in the greater curvature. Gastro-gastrostomy as reconstructions was created extracorporeally or intracorporeally. The intracorporeal reconstructions were represented by delta-shaped anastomosis [11] or by the piercing method [12].
Postoperative management
Postoperative management according to the clinical path was the same between the LDGB1 and LPPG groups. In addition to the above management, we were concerned about the postoperative symptoms and findings in patients after LPPG. When we had a concern that delayed gastric emptying had occurred, close monitoring by X-ray examination, prescription of oral medications, and sufficient dietary instruction were considered. After discharge, all patients who underwent LPPG were administered an anti-acid agent for 1 postoperative month, but not those who underwent LDG.
Evaluation of outcomes
We evaluated the surgery time, blood loss, postoperative morbidities, and mortality in hospital. The postoperative morbidities were evaluated according to the Clavien–Dindo classification and those of grade 2 or higher within 30 days after surgery were recorded. Delayed gastric emptying was defined by the following three conditions: (1) upper abdominal distension; (2) remnant stomach fullness on radiography imaging; and (3) a period of starvation exceeding 24 h. Nutritional parameters, such as changes in the levels of total protein, albumin, and hemoglobin for 5 years and percentage of bodyweight (postoperative bodyweight loss/preoperative bodyweight) (%BW) for 3 years after surgery, were evaluated. To evaluate the total skeletal muscle, including the psoas, paraspinal, and abdominal wall muscles, the skeletal muscle index (SMI) was measured at the level of the third lumbar vertebra with a SYNAPSE VINCENT Volume Analyzer (Fujifilm Medical Co., Tokyo, Japan) [13]. Tissue Hounsfield units (HU) were − 29 to 150 HU for skeletal muscle. The SMI was calculated as follows: SMI = cross-sectional area of the skeletal muscle (cm2)/height(m2). The SMI percentage (postoperative SMI/preoperative SMI) (%SMI) for 3 years after surgery was also evaluated.
In this study, we made three comparisons to identify the significance of LPPG for older patients: first, surgical and endoscopic data; second, postoperative nutritional outcomes between the LDGB1 and LPPG groups; and third, postoperative changes in body weight and SMI between the LDGB1 and LPPG groups. The esophageal reflux and food residue was endoscopically evaluated 1 year after surgery. The severity of esophageal reflux was evaluated by the Los Angeles classification, which defined grade B or higher as positive.
Statistical analysis
Differences in baseline characteristics and outcomes between groups were evaluated. Categorical variables were analyzed using Fisher’s exact test and continuous variables were analyzed using the Mann–Whitney test. The changes in nutritional parameters BW and SMI were compared between LDGB1 and LPPG using the Student’s t-test. Propensity score matching (PSM) was used to improve comparability between the LDGB1 and LPPG groups. Its model was created using logistic regression analysis with the following variables: sex, age, body mass index (BMI), performance status (PS), American Society of Anesthesiologists physical status (ASA-PS) score, tumor size, and histological type. Nearest-neighbor matching was performed with a caliper width equal to 0.25 standard deviations on the logit of the estimated propensity score with the use of a 1:1 matching protocol without replacement. We used the standardized difference to measure covariate balances, whereby an absolute standardized difference of less than 0.2 was considered an adequate balance. Statistical significance was defined as p < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
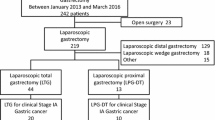
A total of 274 patients were collected in this study. After exclusions, 88 and 44 patients who underwent LDGB1 and LPPG, respectively, for tumors of the same criteria were selected for the study (Fig. 1). Patient characteristics according to the procedures are also shown in Table 1. Significant differences were found between the LDGB1 and LPPG groups in age and tumor size. After adjustment by PSM, 43 patients were eligible in each group and no significant differences were shown between the groups.
Surgical data and postoperative complications
Table 2 shows the comparisons of surgical results between the LDGB1 and LPPG groups after PSM. Although the surgery time was longer in the LPPG group, no significant difference was found compared with the LDGB1 group. No significant differences were also found between the groups regarding blood loss and the overall incidence of postoperative complications. One patient in the LPPG group suffered from anastomotic stricture, but he recovered only by starvation and dietary instruction. Postoperative delayed gastric emptying was not significant but was more frequent in the LPPG group. Patients who suffered from delayed gastric emptying required temporary starvation and oral medication with gastrointestinal motility regulators. Some patients additionally needed the insertion of a nasogastric tube because of severe abdominal fullness or vomiting. Endoscopic findings showed that the incidence of reflux esophagitis after LPPG was similar to that after LDGB1. An amount of food residue that was sufficient to disturb endoscopic evaluations was more frequently observed after LPPG than after LDGB1. The median observation period was 5.7 years (range, 99 days–11.0 years). The 5-year overall survival rates were 100% in the LDGB1 group and 89.6% in the LPPG group (p = 0.359). No recurrence was observed in both groups.
Postoperative nutritional outcomes
Nutritional parameter trends
The trends of nutritional parameters between the LDGB1 and LPPG groups after PSM are represented in Fig. 2. No significant differences were identified in the preoperative levels of total protein, albumin, and hemoglobin between the two groups. The total protein levels after LPPG were significantly higher than those after LDGB1 for 4 postoperative years. The albumin levels after LPPG were consistently higher than after LDGB1 and significant differences were evident at 6 and 12 months. The hemoglobin levels after LDGB1 were higher than those after LPPG for the first 2 years, but conversely, those after LDGB1 were lower than those after LPPG.
Trends of nutritional parameters for long-term observation between the LDGB1 and LPPG groups after matching. For total protein and albumin, the levels in the LPPG group were consistently higher than those in the LDGB1 group for 5 years. Statistically significant differences in total protein levels are shown at 6 postoperative months (P = 0.002), 12 postoperative months (P = 0.005), 24 postoperative months (P = 0.01), 36 postoperative months (P = 0.01), and 48 postoperative months (P = 0.02), and in serum albumin levels at 6 postoperative months (P = 0.04) and 12 postoperative months (p = 0.02). Hemoglobin levels after LDGB1 were higher than those after LPPG for the first 2 years, but those after LDGB1 were conversely lower than those after LPPG. No statistically significant differences are shown. Statistical significance is indicated by asterisks. LDGB1 laparoscopic distal gastrectomy with Billroth I reconstruction, LPPG laparoscopic pylorus-preserving gastrectomy
Trends of %BW
Figure 3a shows the postoperative %BW for 3 years. No significant difference was found in the preoperative level of BW between the two groups (LDGB1 vs LPPG; 55.5 kg vs 56.7 kg; P = 0.624). During the first year after gastrectomy, %BW after LPPG was significantly larger than that after LDGB1, while %BW was almost the same in both groups at the third year after surgery.
Postoperative trends of (a) %BW and (b) %SMI between the LDGB1 and LPPG groups after matching. a During the first year after gastrectomy, %BW after LPPG was significantly larger than that after LDGB1 (LDGB1 vs. LPPG; 89.4% vs. 95.2%, P = 0.004). However, %BWs were almost the same in both groups in the third year after surgery (LDGB1 vs. LPPG; 87.8% vs. 89.2%, P = 0.61). b The %SMI after LPPG was significantly larger than that after LDGB1 at the first year (LDGB1 vs. LPPG; 89.5% vs. 94.5%, P = 0.002). The %SMI after LPPG remained roughly unchanged for the subsequent 2 years. Conversely, the SMI after LDGB1 decreased considerably for the first year and kept decreasing for 3 years (24 months, LDGB1 vs. LPPG; 13.4% vs. 7.4%, P = 0.003, and 36 months, LDGB1 vs. LPPG; 15.5% vs. 8.5%, P = 0.009). Statistical significance is indicated by asterisks. %BW %bodyweight, %SMI %skeletal muscle index, LDGB1 laparoscopic distal gastrectomy with Billroth I reconstruction, LPPG laparoscopic pylorus-preserving gastrectomy
Trends of %SMI
Figure 3b presents the postoperative 3-year trends of %SMI after LDGB1 and LPPG. No significant difference was identified in the preoperative level of SMI between the two groups (LDGB1 vs LPPG; 42.3 cm2/m2 vs 42.5 cm2/m2; P = 0.896). The %SMI after LPPG was significantly larger than that after LDGB1 after the first year. The %SMI after LPPG remained roughly unchanged for the subsequent 2 years. Conversely, the SMI after LDGB1 decreased considerably in the first year and keep decreasing for 3 years. Eventually, the %SMIs were significantly different between the LDGB1 and LPPG groups for 3 years after surgery and the differences became greater every year.
Discussion
In the present study, we evaluated whether LPPG for older patients with cT1N0M0 gastric cancer in the middle third of the stomach has some advantages compared with LDGB1. Three new findings were obtained in this study. First, LPPG was safe for older patients, similar to LDGB1. Second, LPPG had advantages in the postoperative maintenance of nutritional parameters over LDGB1. Third, LPPG was also an advantageous procedure for preventing bodyweight and skeletal muscle losses, especially the latter, compared with LDGB1. Although older age was out of indication for LPPG in many patients, these critical findings suggested that LPPG is also a feasible and advantageous procedure compared with LDG in older patients.
This study showed that LPPG was a safe and viable procedure compared with LDGB1 in older patients as well as in younger patients. The incidence (18.6%) of postoperative complications in the LPPG group was comparable to that in previous studies and acceptable considering the indication for LPPG in older patients. This was probably because delayed gastric emptying, which is one of the distinctive complications of LPPG, had a low incidence, although we exclusively collected older patients who had a decline in their gastrointestinal function caused by aging. The delayed gastric emptying of 6.9% in the LPPG group was relatively high compared with that of the LDGB1 group but was similar to the findings of previous studies [14]. We previously demonstrated that the incidence of delayed gastric emptying increased with age [7]. In another study by Tsujiura et al., we targeted patients aged 75 years or younger and showed that the incidence of delayed gastric emptying was 5.2% after LPPG [4]. Compared with these data, the incidence of 6.9% in the present study was a little higher but was comparable. One of the possible reasons lies in the preservation of hepatic and pyloric branches of the vagus nerve and infrapyloric vein in our techniques. The advantages of vagus nerve and vein preservation in PPG have previously been discussed. Furukawa et al. reported that the incidence of postoperative delayed gastric emptying was not different with and without preservation of the vagus nerve after LPPG [15]. Conversely, Nunobe et al. suggested that preservation of the vagus nerve lowered the incidence of delayed gastric emptying [16]. Furthermore, Kiyokawa et al. reported that preservation of the infrapyloric vein reduced edema of the pyloric cuff and prevented postoperative delayed gastric emptying after LPPG [6]. Thus, preserving the nerve and vessels is probably important to maintain postoperative gastric function, especially in vulnerable older patients, and improves the feasibility and safety of LPPG.
Previous studies identified the postoperative nutritional benefits after LPPG [5, 14]. In fact, the present study verified such benefits of the nutritional parameters, serum total protein, albumin, and hemoglobin for long-term observation. These results suggest that the nutritional outcomes after LPPG are superior to those after LDGB1 even in older patients. However, it is unclear what concrete advantages there are for patients in maintaining serum total protein, albumin, and hemoglobin.
The maintenance of body weight is, however, thought to be the greatest benefit of LPPG because bodyweight loss is a prevalent and noticeable problem after gastrectomy [17]. Nonetheless, this study did not present a persistent benefit of LPPG regarding bodyweight loss. In this study, bodyweight after LDGB1 decreased more than that after LPPG during the first year, but bodyweight after LPPG kept decreasing and was almost the same as that after LDGB1 in the third year. Usually, in LPPG, preservation of the pyloric cuff works as a gastric reservoir and prevents post-gastrectomy syndrome because of the storage capacity. Hosoda et al. reported that those who underwent LPPG rather than LDGB1 felt less dissatisfaction after meals because of reduced dumping syndrome [18]. In older patients, such functional advantages of LPPG can be slightly expected for the maintenance of body weight. The function of the antral cuff and pylorus may be impaired due to aging-related phenomena.
This study clearly presented a muscle-sparing effect of LPPG compared with LDGB1. The evaluation of skeletal muscle loss was previously shown to be significant in terms of its association with increased chemotherapeutic toxicity and postoperative complications as well as physical disability and poor quality of life in patients with various cancers [19,20,21,22]. Several studies reported the postoperative loss of skeletal muscle after gastrectomy [23, 24]. Furthermore, Sugiyama et al. implied that function-preserving gastrectomy had advantages in preventing skeletal muscle loss compared with conventional gastrectomy [25]. Nomura et al. also reported that a smaller remnant stomach resulted in skeletal muscle loss [26]. The present study revealed that skeletal muscle loss after LPPG was not only smaller than that after LDGB1 but also decreased much more slowly from 1 to 3 years after surgery. These results suggest that skeletal muscle mass decreased during the first year after LPPG in older patients, but after that it was maintained, although their bodyweight loss continued. Conversely, LDGB1 showed different patterns consistent with previous reports that body weight decreased during the immediate postoperative period and was maintained over the long-term postoperative course, while the skeletal muscle mass decreased continuously throughout the first 3 years postoperatively [27]. These findings suggest that there is some specific mechanism of maintaining skeletal muscle mass after LPPG in older patients.
One of the possible explanations is that the characteristics of PPG represented by food storage capacity and subsequent prevention of dumping syndrome might contribute to preventing skeletal muscle loss. Dumping syndrome causes postprandial glycemic fluctuations after gastrectomy [28]. We previously showed using continuous glucose monitoring that postprandial blood glucose levels were significantly higher in patients with dumping syndrome [29]. Moreover, these postprandial glycemic changes differ among the types of surgical procedure, and PPG showed mild changes and prevented the glucose spike [30]. Several reports showed associations between these glycemic levels and skeletal muscle mass. Fiolka et al. suggested that hyperglycemia might be one of the factors causing the undesired decrease in skeletal muscle mass [31]. A recent molecular study also showed that hyperglycemia promoted muscle atrophy via the WWP1/LF15 pathway [32]. From these considerations, the mild changes in glucose levels and the suppression of postprandial hyperglycemia after LPPG might contribute to preventing skeletal muscle loss in older patients. Although the further examination is required regarding glucose monitoring of PPG, our results suggested that skeletal muscle loss is unlikely to occur after LPPG, which is one of the greatest advantages after LPPG in older patients because skeletal muscle loss is much more fatal in this patient group.
This study had several limitations. First, this was a single-institutional retrospective study with small sample size. In particular, the study had a smaller number of patients after PSM. Second, patient symptoms were not compared in this study because of its retrospective nature. Symptoms regarding dumping syndrome are one of the important outcomes of PPG. Third, chronological bias should be considered because the surgical indication has changed over time. In fact, we undertook LPPG more aggressively in the later period; therefore, we performed LDGB1 rather than LPPG for older patients, especially from 2014 to 2015. However, the ratios were similar before 2013 and after 2016. As a result, we suggested that there was less chronological deviation in this study. Fourth, differences in the surgery skills among the surgeons were also concerning because LPPG is a slightly more complicated procedure. However, all operations were performed by surgeons who were technically certified or equivalent as an operator or instructive assistant. Finally, the older patients in the LPPG group might be in better health originally, although the characteristics of patients in the LDGB1 and LPPG groups were comparable in this study. The selection bias of surgeons naturally selecting well-conditioned patients for LPPG may have influenced the results of this study. Because large differences in each older individual exist, it may be difficult to generalize the results of this study to all older patients.
In conclusion, LPPG is a safe and feasible procedure for older patients compared with LDGB1. LPPG has great advantages in maintaining postoperative skeletal muscle mass as well as nutritional blood parameters for older patients. Although LPPG takes some time and effort, surgeons should recommend LPPG to older patients with early middle gastric cancer as an advantageous surgery that can spare more skeletal muscle.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Fujishiro M, Yoshida S, Matsuda R, Narita A, Yamashita H, Seto Y. Updated evidence on endoscopic resection of early gastric cancer from Japan. Gastric Cancer. 2017;20(Suppl 1):39–44.
Park DJ, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32(6):1029–36.
Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, et al. Excellent long-term prognosis and favorable postoperative nutritional status after laparoscopic pylorus-preserving gastrectomy. Ann Surg Oncol. 2017;24(8):2233–40.
Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, et al. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19(1):302–11.
Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T. Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg. 2017;402(1):49–56.
Takahashi R, Ohashi M, Hiki N, Makuuchi R, Ida S, Kumagai K, et al. Risk factors and prognosis of gastric stasis, a crucial problem after laparoscopic pylorus-preserving gastrectomy for early middle-third gastric cancer. Gastric Cancer. 2020;23(4):707–15.
Marubashi S, Takahashi A, Kakeji Y, Hasegawa H, Ueno H, Eguchi S, et al. Surgical outcomes in gastroenterological surgery in Japan: report of the National Clinical Database 2011–2019. Ann Gastroenterol Surg. 2021;5(5):639–58.
Japanese Gastric Cancer A. Japanese classification of gastric carcinoma. 3rd English edition. Gastric Cancer. 2011;14(2):101–12
Hiki N, Shimoyama S, Yamaguchi H, Kubota K, Kaminishi M. Laparoscopy-assisted pylorus-preserving gastrectomy with quality-controlled lymph node dissection in gastric cancer operation. J Am Coll Surg. 2006;203(2):162–9.
Kumagai K, Hiki N, Nunobe S, Sekikawa S, Chiba T, Kiyokawa T, et al. Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer. 2015;18(1):183–7.
Ohashi M, Hiki N, Ida S, Kumagai K, Nunobe S, Sano T. A novel method of intracorporeal end-to-end gastrogastrostomy in laparoscopic pylorus-preserving gastrectomy for early gastric cancer, including a unique anastomotic technique: piercing the stomach with a linear stapler. Surg Endosc. 2018;32(10):4337–43.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115–22.
Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259(3):485–93.
Furukawa H, Ohashi M, Honda M, Kumagai K, Nunobe S, Sano T, et al. Preservation of the celiac branch of the vagal nerve for pylorus-preserving gastrectomy: is it meaningful? Gastric Cancer. 2018;21(3):516–23.
Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10(3):167–72.
Climent M, Munarriz M, Blazeby JM, Dorcaratto D, Ramón JM, Carrera MJ, et al. Weight loss and quality of life in patients surviving 2 years after gastric cancer resection. Eur J Surg Oncol. 2017;43(7):1337–43.
Hosoda K, Yamashita K, Sakuramoto S, Katada N, Moriya H, Mieno H, et al. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared with laparoscopy-assisted distal gastrectomy: a cross-sectional postal questionnaire survey. Am J Surg. 2017;213(4):763–70.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–8.
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95(13): e3164.
Chen FF, Zhang FY, Zhou XY, Shen X, Yu Z, Zhuang CL. Role of frailty and nutritional status in predicting complications following total gastrectomy with D2 lymphadenectomy in patients with gastric cancer: a prospective study. Langenbecks Arch Surg. 2016;401(6):813–22.
Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24(4):1037–45.
Okamoto A, Aikou S, Iwata R, Oya S, Kawasaki K, Okumura Y, et al. The type of gastrectomy affects skeletal muscle loss and the long-term outcomes of elderly patients with gastric cancer: a retrospective study using computed tomography images. Surg Today. 2022;52(5):812–21.
Sugiyama M, Oki E, Ando K, Nakashima Y, Saeki H, Maehara Y. Laparoscopic proximal gastrectomy maintains body weight and skeletal muscle better than total gastrectomy. World J Surg. 2018;42(10):3270–6.
Nomura E, Lee SW, Tokuhara T, Nitta T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and the type of reconstruction following distal gastrectomy for gastric cancer: an investigation including total gastrectomy. Jpn J Clin Oncol. 2013;43(12):1195–202.
Asaoka R, Irino T, Makuuchi R, Tanizawa Y, Bando E, Kawamura T, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. 2019;89(1–2):79–83.
Holdsworth CD, Turner D, McIntyre N. Pathophysiology of post-gastrectomy hypoglycaemia. Br Med J. 1969;4(5678):257–9.
Ri M, Nunobe S, Ida S, Ishizuka N, Atsumi S, Makuuchi R, et al. Preliminary prospective study of real-time post-gastrectomy glycemic fluctuations during dumping symptoms using continuous glucose monitoring. World J Gastroenterol. 2021;27(23):3386–95.
Ri M, Nunobe S, Ida S, Ishizuka N, Atsumi S, Hayami M, et al. Postprandial asymptomatic glycemic fluctuations after gastrectomy for gastric cancer using continuous glucose monitoring device. J Gastric Cancer. 2021;21(4):325–34.
Zalejska-Fiolka J, Birková A, Wielkoszyński T, Hubková B, Szlachta B, Fiolka R, et al. Loss of Skeletal Muscle Mass and Intracellular Water as Undesired Outcomes of Weight Reduction in Obese Hyperglycemic Women: A Short-Term Longitudinal Study. Int J Environ Res Public Health. 2022;19(2):1001.
Hirata Y, Nomura K, Senga Y, Okada Y, Kobayashi K, Okamoto S, et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight. 2019;4(4).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by MT. The first draft of the manuscript was written by MT and MO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1964 and its later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terayama, M., Ohashi, M., Makuuchi, R. et al. A continuous muscle-sparing advantage of pylorus-preserving gastrectomy for older patients with cT1N0M0 gastric cancer in the middle third of the stomach. Gastric Cancer 26, 145–154 (2023). https://doi.org/10.1007/s10120-022-01345-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01345-2