Abstract
Background and aim
Endoscopic ultrasonography is a reliable diagnostic modality for determining indications of endoscopic submucosal dissection for early gastric cancer. We aimed to clarify the clinical significance of endoscopic ultrasonography in the invasion depth diagnosis of early gastric cancer.
Methods
We retrospectively assessed 1598 consecutive patients with 2001 early gastric cancers who underwent EUS before ESD or surgery between October 2010 and April 2019 at our institution. Lesions were classified according to endoscopic ultrasonography-determined invasion depth as EUS-M/SM1 (lesions confined to sonographic layers 1 and 2 or lesions with changes in sonographic layer 3; depth, < 1 mm) and EUS-SM2 (lesions with changes in sonographic layer 3; depth, ≥ 1 mm). We evaluated the invasion depth determination accuracy of endoscopic ultrasonography and analyzed the clinicopathological features of misdiagnosed early gastric cancer cases.
Results
The invasion depth determination accuracy was as follows: EUS-M/SM1: pathological T1a/T1b1 early gastric cancer, 97%; EUS-SM2: pathological T1b2 early gastric cancer, 79%. The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value were 95%, 98%, 69%, 97%, and 79%, respectively. In EUS-M/SM1 early gastric cancer, tumor size of ≥ 15 mm, presence of ulceration, and undifferentiated histological type were significantly associated with endoscopic ultrasonography accuracy. In EUS-SM2 early gastric cancer, tumor size of ≥ 30 mm was significantly associated with endoscopic ultrasonography accuracy.
Conclusions
Endoscopic ultrasonography is a useful modality in accurately determining the invasion depth of early gastric cancer before endoscopic submucosal dissection.
Similar content being viewed by others
Introduction
In Japan, gastric cancer is one of the most common cancers and the third most common cause of cancer-related death. There are several options for the treatment of gastric cancer; however, accurate preoperative diagnosis is important to select the appropriate treatment.
Endoscopic submucosal dissection (ESD) is one of the treatments available for gastric cancer; various studies have investigated ESD for gastric cancer [1,2,3,4,5,6,7]. When determining an indication for ESD for early gastric cancer (EGC) according to the Japanese Gastric Cancer Treatment Guidelines 2018 [8], the invasion depth of EGC is one of the most important factors. In the guidelines, the absolute criteria for ESD include differentiated intramucosal cancer without ulceration and differentiated intramucosal cancer with ulceration and tumor size of ≤ 3 cm. The expanded criteria are undifferentiated intramucosal cancer without ulceration with a tumor size of ≤ 2 cm. Additional surgery is also recommended in the current treatment guidelines for gastric cancers with deep submucosal invasion identified in the pathological evaluation after ESD (pT1b2; depth of submucosal invasion, ≥ 500 μm) owing to the risk of lymph node metastasis. Notably, it is important to diagnose whether there is submucosal deep invasion during gastric ESD. Various reports on the diagnostic ability of endoscopic ultrasonography (EUS) have been published. However, most reports have examined the diagnostic ability of EUS that distinguishes T1 from T2 cancer [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]; several reports have examined its diagnostic ability that distinguishes T1a from T1b cancer [24,25,26,27,28,29,30,31] and T1a/T1b1 from T1b2 cancer [31,32,33,34,35,36,37,38,39]. In addition, the number of cases included in each study was up to several hundred at most. In meta-analyses, the number of cases was large; however, the devices used for EUS were not uniform. In this study, we evaluated the ability of EUS with a mini-probe to diagnose submucosal deep invasion and investigated the factors influencing the diagnosis of cancer by comparing the clinicopathological features of cases with an inconsistent depth on preoperative diagnosis by EUS and pathological examination.
Methods
Patients and lesions
We retrospectively assessed 1598 consecutive patients with 2001 EGCs who underwent EUS before ESD or surgery between October 2010 and April 2019 at our institution. There were no patients who underwent ESD and surgery without EUS during this period. Of these 2001 EGCs, 106 EGCs that were insufficient to be diagnosed by EUS for various reasons (e.g., tumor location, ulceration, and elevated type tumor) were excluded. In these cases, the layer structure could not be clearly visualized by EUS, and we could not diagnose the depth of invasion with high confidence. The results of these treatments were retrospectively reviewed. The details of the factors that were insufficient for EUS are shown in Fig. 1. First, the 1895 remaining EGCs were classified into two grades according to the EUS-determined depth of submucosal invasion: EUS-M/SM1 (n = 1734) and EUS-SM2 (n = 161). EUS-M/SM1 was defined as tumors confined to sonographic layers 1 and 2 or lesions with changes in sonographic layer 3 but not deeper than 1 mm. EUS-SM2 was defined as tumors with changes in sonographic layer 3 deeper than 1 mm. Second, each of both groups was subclassified histologically into two grades according to the depth of submucosal invasion: pT1a/T1b1 and pT1b2. pT1a/T1b1 was defined as tumors limited to the mucosal layer (pT1a) or tumors with penetration to the submucosal layer less than 500 µm from the muscularis mucosae (pT1b1); pT1b2 was defined as tumors with penetration of 500 µm or more according to the Japanese Classification of Gastric Carcinoma (15th edition). Finally, we evaluated the accuracy of EUS in determining the depth of tumor invasion and analyzed the clinicopathological features of EGCs that were misdiagnosed by EUS. In addition, the invasion depth of all EGCs was determined by conventional endoscopy (CE) and was performed prior to EUS at our institution. To compare with the diagnostic ability of EUS, EGCs were classified into two grades according to the CE-determined depth of submucosal invasion (CE-M/SM1 and CE-SM2), and each group was subclassified histologically into two grades according to the depth of submucosal invasion: pT1a/T1b1 and T1b2. The depth of invasion diagnosed by CE was performed with comprehensively evaluating several findings such as the concentration of folds of stomach wall, the marginal ridge, the presence or hardening of the stomach wall, and the presence of deformation of the stomach wall due to the amount of air in the stomach [40, 41].
A flow diagram of early gastric cancers included in this study. Patients with gastric cancer who were enrolled in this study were classified based on the EUS diagnosis and pathological diagnosis. EUS endoscopic ultrasonography, ESD endoscopic submucosal dissection, EUS-M/SM1 tumors confined to sonographic layers 1 and 2 or lesion with changes in sonographic layer 3 but no deeper than 1 mm, EUS-SM2 tumors with changes in sonographic layer 3 deeper than 1 mm, pT1a/T1b1 tumors limited the mucosal layer or tumors with penetration into the submucosal layer less than 500 µm from the muscularis mucosae, pT1b2 tumors with penetration of 500 µm or more
Written informed consent was obtained from all patients prior to the procedures and treatment, and the study design was approved by the Ethics Committee of Hiroshima University Hospital (No. 3358).
EUS procedure
The EUS procedures were performed under sedation with midazolam or diazepam. We performed CE to obtain general information on the stomach and magnifying observation with narrow-band imaging and chromoendoscopy in all cases. EUS was performed using 12-MHz and 20-MHz mini-probes (Fujifilm, Saitama, Japan). Deaerated water was instilled to improve the transmission of the ultrasound beam. Acoustic coupling with the gastric wall was achieved by instilling 300–800 mL of deaerated water to the stomach. The entire tumor was observed as much as possible while moving the EUSprobe, and the depth of tumor invasion was evaluated. In the EUS observation, the layer structure of the stomach wall was evaluated as five layers. The invasion depth of the tumor was classified according to the system proposed by Yanai et al. [42]. That is, the tumor remaining in the second layer was described and classified as EUS-M; tumor protruding to the third layer < 1 mm deep, EUS-SM1; and tumor protruding to the third layer ≥ 1 mm deep, EUS-SM2.
Histopathological evaluation
Histopathological examination was based on the Japanese classification of gastric cancer. The specimens resected during ESD and surgical resection were sliced at 2-mm and 5-mm intervals, respectively, after fixation in formalin and were examined under hematoxylin and eosin staining for detailed analysis. Histopathological type, tumor diameter, submucosal invasion depth, lateral and vertical margins, and lymphatic–vascular invasion were evaluated in each slice. Immunohistochemical staining using antibodies against podoplanin (D2–40) was performed to distinguish the small blood vessels from the lymphatic capillaries and to determine the lymphatic invasion. Venous invasion was determined using Elastica van Gieson staining. En bloc resection was defined as resection in a single piece. The histopathological type was classified as the differentiated type (well- or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma) and the undifferentiated type (poorly differentiated tubular adenocarcinoma, mucinous adenocarcinoma, or signet-ring cell carcinoma) according to the 15th edition of the Japanese Classification of Gastric Carcinoma [43].
Investigated variables
The following clinicopathological variables were evaluated for each group: sex, age, tumor location, macroscopic type, tumor size, presence of ulceration, main histopathological type (differentiated type or undifferentiated type), and submucosal invasion depth. We compared these variables between the two groups and analyzed which factors influenced the discrepancy between the diagnoses by EUS and by pathological examination.
Statistical analysis
The chi-square and Fisher’s exact tests were used to evaluate the associations among the various categorical variables in the intergroup comparisons of the clinicopathological characteristics. p values of < 0.05 were considered significant. All analyses were performed using the JMP Pro 15 software (SAS Institute, Cary, NC, USA).
Results
Clinicopathological features of the patients and EGCs
The clinicopathological features of the patients and lesions in this study are shown in Table 1. There were 1102 men in this study, and the mean age of the patients was 70.3 ± 10.1 years. Eight hundred forty-eight EGCs (44%) were located in the lower third of the stomach, and one thousand one hundred twenty-seven (59%) were of the depressed type. The mean tumor size was 17.8 ± 12.7 mm; 202 lesions (11%) were accompanied by ulceration, and 187 lesions (10%) were of the undifferentiated type. A total of 1710 lesions (90%) were diagnosed as pT1a/T1b1 lesions and 185 lesions (10%) as pT1b2 lesions by pathological examination. Incidentally, there were only three lesions of VM + among the ESD cases without residual cancer after additional surgical resection. Thus, it was possible to diagnose T1b2 cancer pathologically. Therefore, we decided that there was no need to exclude these lesions.
Accuracy of EUS and CE diagnosis
The accuracy of the diagnosis of the invasion depth is shown in Table 2. Of all 1895 lesions, 1734 lesions (92%) were diagnosed as M/SM1 by EUS (EUS-M/SM1). Of these, 1676 lesions (97%) were diagnosed as T1a/T1b1 cancer, while 58 lesions (3%) were diagnosed as T1b2 cancer based on the histopathological findings (underestimation group). A total of 161 lesions (8%) were diagnosed as SM2 by EUS (EUS-SM2). Of these, 127 lesions (79%) were diagnosed as T1b2 cancer, while 34 lesions (21%) were diagnosed as T1a/T1b1 cancer based on the histopathological findings (overestimation group). The overall diagnostic accuracy, sensitivity, and specificity of EUS for diagnosing submucosal cancer were 95%, 98%, and 69%, respectively. The positive and negative predictive values were 97% and 79%, respectively. The accuracy of the diagnosis of the invasion depth by CE is shown in Table S1. Of all 1895 lesions, 1759 lesions (93%) were diagnosed as M/SM1 by CE (CE-M/SM1). Of these, 1661 lesions (94%) were diagnosed as T1a/T1b1 cancer, while 98 lesions (6%) were diagnosed as T1b2 cancer based on the histopathological findings. A total of 136 lesions (7%) were diagnosed as SM2 by CE (CE-SM2). Of these, 87 lesions (64%) were diagnosed as T1b2 cancer, while 49 lesions (36%) were diagnosed as T1a/T1b1 cancer based on the histopathological findings. The overall diagnostic accuracy, sensitivity, and specificity of EUS for diagnosing submucosal cancer were 92, 97, and 47%, respectively. The positive and negative predictive values were 94 and 64%, respectively. Compared with the accuracy of the diagnosis based on EUS, EUS was associated with 3% higher accuracy, 1% higher sensitivity, and 22% higher specificity than those associated with CE. The p values were as follows: accuracy, p ≤ 0.01; sensitivity, p = 0.09; specificity, p < 0.01.
Clinicopathological features of EUS-M/SM1 gastric cancer
The clinicopathological features of EUS-M/SM1 gastric cancer are shown in Table 3. The proportion of the tumors of the depressed type and those with a diameter of ≥ 15 mm, presence of ulceration, and undifferentiated-type histology was greater in the pT1b2 group than in the pT1a/T1b1 group: depressed type, 57% (960/1676) versus 76% (44/58), p < 0.01; tumor size of ≥ 15 mm, 45% (754/1676) versus 74% (43/58), p < 0.01; presence of ulceration, 7% (114/1676) versus 28% (16/58), p < 0.01; and undifferentiated-type histology, 7% (120/1676) versus 24% (14/58), p < 0.01.
Table 4 shows the factors related to the misdiagnosis of M/SM1 by EUS in pathological T1b2 cancer. In the multivariate analysis, tumor diameter of ≥ 15 mm, presence of ulceration, and undifferentiated-type histology were found to be the independent risk factors for the misdiagnosis of M/SM1 by EUS in pathological T1b2 cancer (tumor size of ≥ 15 mm: odds ratio (OR) = 2.98, 95% confidence interval (95% CI) = 1.62–5.47; presence of ulceration: OR = 3.85, 95% CI = 2.05–7.26; undifferentiated-type histology: OR = 2.93, 95% CI = 1.48–5.79).
Clinicopathological features of EUS-SM2 gastric cancer
The clinicopathological features of EUS-SM2 gastric cancer are shown in Table 5. The proportion of the tumors with a diameter of 30 mm and presence of ulceration was greater in the pT1a/T1b1 group than in the pT1b2 group: tumor size of ≥ 30 mm, 56% (19/34) versus 33% (42/127), p = 0.02; presence of ulceration, 65% (22/34) versus 39% (50/127), p < 0.01.
Table 6 shows the factors related to the misdiagnosis of SM2 by EUS in pathological T1a/T1b1 cancer. In the multivariate analysis, tumor diameter of ≥ 30 mm and presence of ulceration were found to be the risk factors for the misdiagnosis of SM2 by EUS in pathological T1a/T1b1 cancer (tumor size of ≥ 30 mm: OR = 2.34, 95% CI = 1.05–5.20; presence of ulceration: OR = 2.75, 95% CI = 1.16–6.57).
Clinicopathological features of the lesions misdiagnosed by EUS
The clinicopathological features of the lesions misdiagnosed as M/SM1 by EUS in pathological T1b2 cancer are shown in Supplementary Table S2. Twenty lesions (34%, 34/58) were diagnosed as non-solid poorly differentiated adenocarcinoma (por2) or signet-ring cell carcinoma (sig); seven tumors (12%) showed type C infiltrative growth (Inf C) in the pathological examination; and twelve lesions (21%) had rich vessels in the submucosal layer.
In seven lesions, the depth of tumor invasion was less than 600 µm. Based on the images obtained during CE and EUS in the misdiagnosed cases, 16 lesions (28%) were located near the esophagogastric junction or pylorus. Thus, the lesions in these cases might be poorly observed owing to tumor localization.
The clinicopathological features of the lesions misdiagnosed as SM2 by EUS in pathological T1a/T1b1 cancer are shown in Supplementary Table S3. There were ten tumors with lymphoid follicles in the submucosal layer. (Figs. 2, 3).
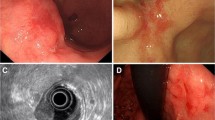
Correct diagnosis of invasion depth of EGC. a White light image of depressed type EGC showing 10 mm in diameter at the corpus of the stomach. b Chromoendoscopic image with indigo carmine dye spraying. c Endoscopic ultrasonography image of the lesion, showing the hypoechoic mass protruding to the submucosal layer. ESD as a total excisional biopsy was performed due to concomitant disease and old age. d Pathological diagnosis was tub1, pT1b2 (SM 1500 µm), ly(−), v(−), HM0, VM0
Incorrect diagnosis of invasion depth of early gastric cancer. a White light image of EGC showing 30 mm in diameter at the corpus of the stomach. b Chromoendoscopic image with indigo carmine dye spraying. c Endoscopic ultrasonography image of the lesion, showing the hypoechoic mass protruding to the submucosal layer. Surgical operation was performed. d Pathological diagnosis was tub1, pT1a, ly(−), v(−). There were lymphoid follicles under the muscularis mucosae and it might be the cause of incorrect diagnosis
Discussion
Our study revealed that the accuracy of EUS for the diagnosis of invasion depth was high in EGC. As reported in several previous studies, EUS had a high sensitivity and a low specificity for T1a/T1b1 gastric cancer [24, 38, 44]. Further, the accuracy of discriminating mucosal cancer from submucosal cancer by CE alone was reported to be 62–80% [32], and there was no significant difference in the diagnostic ability between CE and EUS [24, 38, 44]. In this study, there was no significant difference in sensitivity between CE and EUS, but there was a significant difference in accuracy specificity. This result suggested that T1b2 cancer might be under-diagnosed by only CE observation without EUS and that the number of additional surgical resections after ESD for patients with T1b2 cancer might be increased. However, these were the results of observation with CE alone or EUS alone. The combined use of CE and EUS was reported to show a significantly higher accuracy for the diagnosis of invasion depth in EGC in comparison to CE alone or EUS alone [38]. EUS was very useful for the preoperative diagnosis of invasion depth of EGC when the examination was performed by a trained operator and when the case with a poor study was excluded. Furthermore, the use of the mini-probe device allowed us to perform EUS without changing the scope after making observations with CE. In this study, EUS was performed using a mini-probe device. Mini-probe EUS has been reported to be able to assess the depth of cancer invasion more precisely in small and superficial cancers compared to the conventional EUS (radial and convex types) because the former uses a higher frequency (7.5–30 MHz) [45, 46]. Thus, as observed in this study, the application of EUS using a mini-probe device is reasonable for diagnosing whether gastric cancer is an indication for ESD. Regarding the diagnostic ability of EUS to distinguish between T1a and T1b EGCs, the reported sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) in a previous meta-analysis were 87%, 75%, 3.4, and 0.17, respectively [27]. However, there was no distinction between the mini-probe and conventional types in this meta-analysis. In another study that used only mini-probe EUS, the accuracy, sensitivity, and specificity were 84%, 95%, and 43%, respectively [30]. In another meta-analysis, the diagnostic ability of mini-probe EUS to distinguish between T1a and T1b1 cancers was reported as follows: sensitivity of 92%, specificity of 69%, PLR of 2.83, and NLR of 0.13 [31]. In this study, the diagnostic ability to distinguish between T1a/T1b1 and T1b2 cancers was analyzed; the sensitivity, specificity, PLR, and NLR observed were 98%, 69%, 3.2, and 0.06, respectively. This result was comparable with the diagnostic ability of EUS for differentiation between T1a and T1b cancers and between T1a and T1b1 cancers.
Several studies have reported that the tumor location [21,22,23,24, 29, 32, 47], macroscopic type [23, 31, 37], tumor size [22, 29, 31, 32, 47], presence of ulceration [23, 25, 31, 34, 35, 46,47,48], and histological type [29, 31,32,33,34, 47] are the clinicopathological factors of tumors that affect the accuracy of EUS.
Regarding the location of lesions, there have been various reports on the influence of lesion location on the diagnostic ability of EUS. It was reported that the accuracy of EUS decreased in cases with lesions in the upper third of the stomach, including the cardia [22, 24, 32, 37, 47]. Whether the tumor location was the factor associated with the overestimation or underestimation of the invasion depth of tumors varied among studies. One reason for the decreased accuracy of EUS may be the difference in the thickness of the stomach layers, presence of fibrosis, or blood vessels surrounding the tumor [22, 32, 37]. Other reasons may be technical difficulties associated with scanning the lesions in the upper third of the stomach and difficulty in achieving the necessary pool of nonaerated water in the upper third of the stomach, especially in the lesser curvature [21, 47]. In this study, there was no significant difference in the diagnostic ability of EUS depending on the location of the lesions. This might be because lesions at the cardia were excluded because of poor studies.
Our study showed that the presence of ulceration and a large tumor size were the factors related to the misdiagnosis of M/SM1 by EUS in pathological T1b2 cancer (underdiagnosis) and of SM2 by EUS in pathological T1a/T1b1 cancer (overdiagnosis). There have been several reports that the tumor size affects the accuracy of EUS [16, 22, 23, 25, 29, 31, 32, 47, 49, 50]. Previous studies have reported that a large tumor size was one of the risk factors for overdiagnosis of the depth of tumor invasion; however, no clear reason has been stated [16, 29, 49, 50]. As we speculate, the lesions might not extend even if the deaerated water is stored in cases of large tumors. Thus, the lesion and gastric wall might be scanned “diagonally” by EUS, and the depth of tumor invasion might be overdiagnosed by EUS. Conversely, the reason for the underestimation in the cases of large tumors might be the fact that the probe could not be properly placed at the site where cancer infiltrated to the submucosal deep layer. Similar to previous findings, the accuracy of EUS significantly decreased to 55–78% in EGC with ulceration [25, 31, 34, 48]. In this study, the accuracy of EUS was 95%. However, the accuracy of EUS in the presence of ulceration was significantly greater than that in the absence of ulceration (97% versus 81%, p < 0.01). The reason for the decrease in the accuracy of EUS in the presence of ulceration might be that submucosal fibrosis due to ulceration was difficult to distinguish from cancer, as reported in several reports [30, 32, 35, 36, 47]. Undifferentiated-type tumors tend to have diffuse or vesicular invasion of tumor cells to the submucosal layer of the gastric wall compared to differentiated-type tumors [34, 51, 52]. Thus, EUS could not visualize these microinvasions and might underestimate the depth of cancer invasion.
The limitations of our study need to be acknowledged. The results were obtained from a retrospective assessment based on the medical records of patients undergoing gastric ESD or surgery at a single cancer center in Japan. As such, selection bias could not be denied. In addition, the data were collected not from the EUS image review but from the written text results. Therefore, gross endoscopic images should have been considered for the depth of invasion evaluation. Thus, prospective multicenter nationwide studies are needed to more precisely evaluate the clinical validity of EUS in patients with EGC. In this study, 106 lesions (5%) were excluded because of insufficient observation by EUS owing to reasons, such as the location or macroscopic type of lesions. Thus, there were cases in which it was difficult to diagnose the depth of invasion of EGC using EUS alone because of a tall prominent lesion or a location, such as the esophagogastric junction. In such cases, other modalities, such as CE and contrast radiography of the upper gastrointestinal tract, should be performed actively.
In conclusion, EUS is a useful method for accurately determining the invasion depth of EGCs before ESD. However, attention should be paid not to underestimate the invasion depth of EGCs with a large tumor size, an ulceration, or an undifferentiated-type histology and not to overestimate the invasion depth of EGCs with a large tumor size and an ulceration.
References
Sanomura Y, Oka S, Tanaka S, Noda I, Higashiyama M, Chayama K, et al. Clinical validity of endoscopic submucosal dissection for submucosal invasive gastric cancer: a single-center study. Gastric Cancer. 2012;15(1):97–105.
Sanomura Y, Oka S, Tanaka S, Higashiyama M, Yoshida S, Chayama K, et al. Predicting the absence of lymph node metastasis of submucosal invasive gastric cancer: expansion of the criteria for curative endoscopic resection. Scand J Gastroenterol. 2010;45(12):1480–7.
Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Chayama K, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38(10):996–1000.
Higashimaya M, Oka S, Tanaka S, Numata N, Sanomura Y, Chayama K, et al. Endoscopic submucosal dissection for residual early gastric cancer after endoscopic submucosal dissection. Gastrointest Endosc. 2013;77(2):298–302.
Higashimaya M, Oka S, Tanaka S, Sanomura Y, Yoshida S, Chayama K, et al. Outcome of endoscopic submucosal dissection for gastric neoplasm in relationship to endoscopic classification of submucosal fibrosis. Gastric Cancer. 2013;16(3):404–10.
Sanomura Y, Oka S, Tanaka S, Numata N, Higashiyama M, Chayama K, et al. Continued use of low-dose aspirin does not increase the risk of bleeding during or after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2014;17(3):489–96.
Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Chayama K, et al. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28(2):639–47.
Association Japanese Gastric Cancer (2018) Japanese gastric cancer treatment guidelines 2018 (5th edition) Japanese, 5. Kanehara & CO. Ltd, Tokyo.
Costa JM, Gonçalves B, Miguel Gomes M, Fernandes D, Gonçalves R, Soares JB. Accuracy of endoscopic ultrasound in gastric adenocarcinoma patient selection for neoadjuvant therapy. United Eur Gastroenterol J. 2019;7(2):278–86.
Serrano OK, Huang K, Ng N, Yang J, Friedmann P, Kennedy TJ, et al. Correlation between preoperative endoscopic ultrasound and surgical pathology staging of gastric adenocarcinoma: a single institution. J Surg Oncol. 2016;113(1):42–5.
Yan Y, Wu Q, Li ZY, Bu ZD, Ji JF. Endoscopic ultrasonography for pretreatment T-staging of gastric cancer: an in vitro accuracy and discrepancy analysis. Oncol Lett. 2019;17(3):2849–55.
Merkow RP, Herrera G, Goldman DA, Gerdes H, Schattner MA, Coit DG, et al. Endoscopic ultrasound as a pretreatment clinical staging tool for gastric cancer: association with pathology and outcome. Ann Surg Oncol. 2017;24(12):3658–66.
Guo T, Yao F, Yang AM, Li XY, Zhong DR, Lu XH, et al. Endoscopic ultrasound in restaging and predicting pathological response for advanced gastric cancer patients after neoadjuvant chemotherapy. Asia-Pac J Clin Oncol. 2014;10:e28–e32.
Han C, Lin R, Shi H, Liu J, Qian W, Hou X, et al. The role of endoscopic ultrasound on the preoperative T staging of gastric cancer. Medicine. 2016;95(36):e4580.
Park CH, Park JC, Kim EH, Jung DH, Chung H, Lee YC, et al. Learning curve for EUS in gastric cancer T staging by using cumulative sum analysis. Gastrointest Endosc. 2015;81(4):898–905.
Hwang SW, Lee DH. Is endoscopic ultrasonography still the modality of choice in preoperative staging of gastric cancer? O World J Gastroenterol. 2014;20(38):13775–82.
Lee HH, Lim CH, Park JM, Cho YK, Song KY, Park CH, et al. Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol. 2012;15(10):190.
Jürgensen C, Brand J, Nothnagel M, Arlt A, Neser F, Hampe J, et al. Prognostic relevance of gastric cancer staging by endoscopic ultrasound. Surg Endosc. 2013;27(4):1124–9.
Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Pawlik TM, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg. 2015;220(1):48–56.
Nie RC, Yuan SQ, Chen XJ, Chen S, Xu LP, Chen YB, et al. Endoscopic ultrasonography compared with multidetector computed tomography for the preoperative staging of gastric cancer: a meta-analysis. World J Surg Oncol. 2017;15(1):113.
Ganpathi IS, So JB, Ho KY. Endoscopic ultrasonography for gastric cancer. Does it influence treatment? Surg Endosc. 2006;20(4):559–62.
Razavi SM, Khodadost M, Sohrabi M, Keshavarzi A, Zamani F, Ranjbaran M, et al. Accuracy of endoscopic ultrasonography for determination of tumor invasion depth in gastric cancer. Asian Pac J Cancer Prev. 2015;16(8):3141–5.
Park JM, Ahn CW, Yi X, Hur H, Lee KM, Han SU, et al. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11(2):109–15.
Park CH, Park JC, Chung H, Shin SK, Lee SK, Lee YC. A specific role of endoscopic ultrasonography for therapeutic decision-making in patients with gastric cardia cancer. Surg Endosc. 2016;30(10):4193–9.
Park JS, Kim H, Bang B, Kwon K, Shin Y. Accuracy of endoscopic ultrasonography for diagnosing ulcerative early gastric cancers. Medicine. 2016;95(30):e3955.
Kim J, Kim SG, Chung H, Lim JH, Choi JM, Jung HC, et al. Clinical efficacy of endoscopic ultrasonography for decision of treatment strategy of gastric cancer? Surg Endosc. 2018;32(9):3789–97.
Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2:CD009944.
Ikoma N, Lee JH, Bhutani MS, Ross WA, Weston B, Badgwell BD, et al. Preoperative accuracy of gastric cancer staging in patient selection for preoperative therapy: race may affect accuracy of endoscopic ultrasonography. J Gastrointest Oncol. 2017;8(6):1009–177.
Kim JH, Song KS, Youn YH, Lee YC, Cheon JH, Chung JB, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66(5):901–8.
Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34(12):973–8.
Pei Q, Wang L, Pan J, Ling T, Lv Y, Zou X. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: a meta-analysis. J Gastroenterol Hepatol. 2015;30(11):1566–73.
Watari J, Ueyama S, Tomita T, Ikehara H, Hori K, Miwa H, et al. What types of early gastric cancer are indicated for endoscopic ultrasonography staging of invasion depth? World J Gastrointest Endosc. 2016;8(16):558–67.
Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43(4):318–22.
Yoshida S, Tanaka S, Kunihiro K, Mitsuoka Y, Hara M, Chayama K, et al. Diagnostic ability of high-frequency ultrasound probe sonography in staging early gastric cancer, especially for submucosal invasion. Abdom Imaging. 2005;30(5):518–23.
Mandai K, Yasuda K. Accuracy of endoscopic ultrasonography for determining the treatment method for early gastric cancer. Gastroenterol Res Pract. 2012;2012:245390.
Yamamoto S, Nishida T, Kato M, Inoue T, Hayashi Y, Takehara T, et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol Res Pract. 2012;2012:194530.
Tsuzuki T, Okada H, Kawahara Y, Nasu J, Takenaka R, Yamamoto K, et al. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Med Okayama. 2011;65(2):105–12.
Tsujii Y, Kato M, Inoue T, Yoshii S, Nagai K, Takehara T, et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc. 2015;82(3):452–9.
Kim TY, Yi NH, Hwang JW, Kim JH, Kim GH, Kang MS. Morphologic pattern analysis of submucosal deformities identified by endoscopic ultrasonography for predicting the depth of invasion in early gastric cancer. Surg Endosc. 2019;33(7):2169–80.
Nagahama T, Yao K, Imamura K, Kojima T, Ohtsu K, Iwashita A, et al. Diagnostic performance of conventional endoscopy in the identification of submucosal invasion by early gastric cancer: the “non-extension sign” as a simple diagnostic marker. Gastric Cancer. 2017;20(2):304–13.
Takeda T, So S, Sakurai T, Nakamura S, Yoshikawa I, Yao K. Learning effect of diagnosing depth of invasion using non-extension sign in early gastric cancer. Digestion. 2020;101(2):191–7.
Yanai H, Matsubara Y, Kawano T, Okamoto T, Hirano A, Okita K, et al. Clinical impact of strip biopsy for early gastric cancer. Gastrointest Endosc. 2004;60(5):771–7.
Association Japanese Gastric Cancer (2017) Japanese classification of gastric carcinoma (October 2017 [The 15th Edition]) Japanese, 15. Kanehara & CO. Ltd, Tokyo.
Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–13.
Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008;12(2):256–62.
Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Is endoscopic ultrasonography indispensable in patients with early gastric cancer prior to endoscopic resection? Surg Endosc. 2010;24(12):3177–85.
Okada K, Fujisaki J, Kasuga A, Omae M, Yoshimoto K, Takahashi H, et al. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded- indication criteria for endoscopic submucosal dissection. Surg Endosc. 2011;25(3):841–8.
Akashi K, Yanai H, Nishikawa J, Satake M, Fukagawa Y, Sakaida I, et al. Ulcerous change decreases the accuracy of endoscopic ultrasonography diagnosis for the invasive depth of early gastric cancer. Int J Gastrointest Cancer. 2006;37(4):133–8.
Akahoshi K, Chijiwa Y, Hamada S, Sasaki I, Nawata H, Okabe H, et al. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc. 1998;48(5):470–6.
Saadany SE, Mayah W, Kalla FE, Atta T. Endoscopic ultrasound staging of upper gastrointestinal malignancies. Asian Pac J Cancer Prev. 2016;17(5):2361–7.
Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer. 1998;1(1):31–50.
Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32(Suppl 2):329–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement/human rights statement and informed consent
All study procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Written informed consent or substitute for it was obtained from all patients for inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuroki, K., Oka, S., Tanaka, S. et al. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer 24, 145–155 (2021). https://doi.org/10.1007/s10120-020-01100-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01100-5