Abstract
Background
Oral fluoropyrimidine S-1 contains tegafur, which is metabolized to 5-fluorouracil by cytochrome P450 2A6 (CYP2A6). We here examined associations between CYP2A6 polymorphisms and treatment outcomes of adjuvant S-1 in gastric cancer patients.
Methods
Patients received adjuvant S-1 (40 mg/m2 twice daily, days 1–28, every 6 weeks for eight cycles) after curative surgery for pathological stage II–III gastric cancer. We analyzed the wild-type allele (W) (CYP2A6*1) and four variant alleles (V) (CYP2A6*4, *7, *9, *10) that abolish or reduce this enzyme activity.
Results
Patients (n = 200) were enrolled between November 2007 and July 2013 with the following clinical characteristics: median age, 57 years (range, 32–83 years); 128 men, 72 women. With a median follow-up of 46.4 months, the 3-year relapse-free survival (RFS) and overall survival (OS) rates were 83.1 % (95 % CI, 77.7–88.5 %) and 94.8 % (95 % CI, 91.6–98.0 %), respectively. Genotype distributions were as follows: W/W (n = 49, 24.5 %), W/V (n = 94, 47.0 %), and V/V (n = 57, 28.5 %). Overall toxicity did not differ according to genotype for any grade (p = 0.612) or grade ≥3 (p = 0.143). However, RFS differed significantly according to CYP2A6 genotype. The 3-year RFS rates were 95.9 % for W/W, 83.1 % for W/V, and 72.5 % for V/V (p = 0.032). Carriers of W/V and V/V genotypes had a poorer RFS with a hazard ratio of 3.41 (95 % CI, 1.01–11.52; p = 0.049) and 4.03 (95 % CI, 1.16–13.93; p = 0.028), respectively, compared with the W/W genotype.
Conclusions
CYP2A6 polymorphisms are not associated with toxicity of S-1 chemotherapy, but correlate with the efficacy of S-1 in the adjuvant setting for gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is a leading cause of cancer-related deaths worldwide [1]. The highest incidences of GC occur in Eastern Asia, including Korea, Eastern Europe, and South America. In Korea, GC is a major health issue and represents the second leading cause of cancer [2]. In patients with localized GC, complete surgical resection represents the mainstay treatment and only available curative option.
Recently, large-scale randomized phase III clinical trials have shown that adjuvant chemotherapy can improve survival in patients with curatively resected GC [3–6]. The Japanese Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) study showed that adjuvant S-1 chemotherapy, following curative gastrectomy with D2 dissection, increased both relapse-free survival (RFS) and overall survival (OS) in patients with stage II or III GC [5]. Following the ACTS-GC trial, adjuvant chemotherapy with S-1 has been widely used in Asian countries and has shown consistent efficacy and safety profiles in patients with GC [7–9].
An oral fluoropyrimidine, S-1 contains tegafur (FT), 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate (Oxo) at molar ratios of 1:0.4:1 [10]. FT is a prodrug that gradually releases 5-fluorouracil (5-FU) in a process that is mainly catalyzed by the liver microsomal enzyme cytochrome P450 2A6 (CYP2A6) [11]. Genetic polymorphisms in the CYP2A6 gene have been associated with variations in enzyme activity; CYP2A6*2, *4, *5, and *20 exhibit no enzyme activity, whereas CYP2A6*6, *7, *9, *10, *11, *12, *17, *18, and *19 yield enzymes with reduced activity (see http://www.cypalleles.ki.se). Previous studies have described an association between CYP2A6 polymorphisms and the pharmacokinetic profile of S-1, with many CYP2A6 variants being associated with reduced metabolism of FT to 5-FU [12–15]. Kaida and colleagues reported that CYP2A6*4 results in reduced plasma 5-FU concentrations and increases the area under the concentration–time curve (AUC) and C max for FT in non-small cell lung cancer patients treated with S-1 alone or in combination with cisplatin [12]. Fujita and colleagues also showed that FT clearance was significantly lower in patients with two variant CYP2A6 alleles versus individuals with wild-type or one variant allele who were treated with S-1 for solid tumors [13]. Similarly, in two recent studies that evaluated the use of S-1 plus oxaliplatin in biliary tract cancer and S-1 plus oxaliplatin and irinotecan for GC or colorectal cancer, patients with CYP2A6 variant alleles had a significantly lower AUC and/or C max for 5-FU and the metabolic ratio (exposure ratio of 5-FU to tegafur) versus patients with a wild-type genotype [14, 15]. Based on associations with these pharmacokinetic differences and CYP2A6 polymorphisms, we hypothesized that CYP2A6 genotypes affect the clinical outcomes of patients treated with adjuvant S-1 chemotherapy for curatively resected GC.
Patients and methods
Study design and treatment
This retrospective study included 200 patients who had received adjuvant S-1 chemotherapy following curative gastrectomy with D2 lymph node dissection for GC at the Asan Medical Center between October 2007 and May 2013. All patients met the following eligibility criteria: pathological stage II–III GC, as defined by the American Joint Committee on Cancer (AJCC) staging system, 7th edition; Eastern Cooperative Oncology Group (ECOG) performance status ≤2; age ≥18 years; no coexisting malignancy or severe comorbidity that might influence the treatment dose and schedule; no prior chemotherapy for GC; and an adequate amount of peripheral blood for analysis of CYP2A6 polymorphisms.
Adjuvant S-1 was initiated from 3 to 6 weeks after surgery. If there was no evidence of tumor recurrence or unacceptable toxicity, oral S-1 (40 mg/m2) was administered twice daily for 4 weeks followed by 2 weeks of rest in a 6-week cycle for a maximum of eight cycles. The S-1 dose was proportional to the body surface area, based on which the actual dosing of S-1 that patients received ranged from 80 to 160 mg/day. Patients with a body surface area of more than 2.00 m2 received 160 mg daily. If patients had grade 3–4 hematological adverse events or grade 2–4 nonhematological adverse events, the S-1 dose was reduced at the discretion of the physician.
The institutional review board of Asan Medical Center approved this study and all patient subjects provided written informed consent.
Evaluation of efficacy and adverse events
Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE version 3.0). Physical and blood examinations of patients included a complete blood cell count with differentials, serum chemistry tests, and electrolyte measurements, which were performed every 6 weeks during treatment, every 3 months during the first 3 years after surgery, and then every 6 months thereafter. Abdominopelvic computed tomography (CT) scans and chest X-rays were performed every 6 months for 5 years and annually thereafter. Esophagogastroduodenoscopy was performed annually.
CYP2A6 genotyping
We extracted genomic DNA using a DNA preparation kit (Qiagen, Hilden, Germany) from 5 ml peripheral blood. We detected CYP2A6*4, *7, *9, and *10 variants, which affect CYP2A6 activity or expression and are common variant alleles in Asian populations, along with the wild-type allele (CYP2A6*1), as previously described [16]. Briefly, polymerase chain reaction (PCR)-restriction fragment length polymorphism assessments and sequencing were used to determine three polymorphic sites (−48T>G, 6558T>C, and 6600G>T) and to identify deletions of the CYP2A6 gene. PCR reactions were performed using a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA ). Sequencing used an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit, version 3.0, with an automated ABI Prism 3100 Genetic Analyzer (Applied Biosystems).
Statistical analysis
Discrete data were compared using Pearson’s chi-square test or Fisher’s exact test; quantitative data were compared using one-way analysis of variance (ANOVA) or the Kruskal–Wallis test. RFS was defined as the time from surgery to recurrence or death from any cause, and OS was defined as the time between surgery and death from any cause. The Kaplan–Meier method and log-rank test were used to estimate and compare survival distributions, respectively. Multivariate analysis of contributing factors for adverse events (binary logistic regression) and survival (Cox regression) were compared. All variables with a p value of 0.2 or less by univariate analysis were included in the multivariate analysis; two-sided p values less than 0.05 were considered to denote statistically significant differences.
Results
Patient characteristics and CYP2A6 genotypes
A total of 200 patients were enrolled in this study between November 2007 and July 2013. The median patient age was 57 years (range, 32–83 years), and most (98 %) of these patients exhibited a good performance status (ECOG 0 or 1; Table 1). The distributions of allelic frequencies were 0.45 for CYP2A6*1, 0.13 for CYP2A6*4, 0.14 for CYP2A6*7, 0.01 for CYP2A6*8, 0.21 for CYP2A6*9, and 0.04 for CYP2A6*10, which were similar to those reported in previously published studies of Asian populations [17–20]. To test the effects of CYP2A6 polymorphisms on the treatment outcomes of adjuvant S-1 treatment, we combined CYP2A6*4, CYP2A6*7, CYP2A6*9, and CYP2A6*10 in a variant allele category. Because CYP2A6*8 encodes an enzyme with full enzymatic activity, it was considered to be the wild-type allele [21]. We assigned patients who harbored CYP2A6*1/*1 or *1/*8 variations to a wild-type/wild-type (W/W) group, those with CYP2A6*1/*4, *1/*7, *1/*9, or *1/*10 to a wild-type/variant (W/V) group, and those with two variant alleles to a variant/variant (V/V) group. The patient genotypic distributions were as follows: 24.5 % W/W (n = 49), 47.0 % W/V (n = 94), and 28.5 % V/V (n = 57). There were no significant differences in baseline characteristics between the groups of patients distinguished by CYP2A6 genotype (Table 1). The median follow-up duration was 46.4 months (range, 17.1–91.0 months), and the median duration of S-1 treatment was 11.3 months (range, 2.8–13.7 months) in the W/W group, 11.3 months (range, 1.4–14.3 months) in the W/V group, and 11.4 months (range, 4.2–14.0 months) in the V/V group (p = 0.146). The median relative dose intensity of S-1 was 0.96 (range, 0.54–1.00) in the W/W group, 0.99 (range, 0.47–1.00) in the W/V group, and 0.92 (range, 0.63–1.00) in the V/V group (p = 0.329). The median total dose of S-1 was 23,318, 26,297, and 24,640 mg in the W/W, W/V, and V/V groups, respectively (p = 0.656).
Associations between CYP2A6 genotypes and adverse events
All 200 patients were evaluated for both hematological and nonhematological toxicities. Table 2 shows adverse events that occurred during the treatment period. Treatments were considered well tolerated, and there were no grade 4 toxicities or treatment-related deaths. Neutropenia (11.5 %) and abdominal pain (9.0 %) were the most common grade 3 hematological and nonhematological adverse events, respectively. No CYP2A6 polymorphism showed a significant association with hematological or nonhematological adverse events (Table 3). The incidence of hematological adverse events of grade ≥3 was 10.2 %, 14.9 %, and 10.5 % in the W/W, W/V, and V/V groups, respectively (p = 0.628), whereas the incidence of nonhematological adverse events of grade ≥3 was 12.2 %, 24.5 %, and 21.1 %, respectively (p = 0.227).
Renal impairment [creatinine clearance (Ccr) calculated by the Cockroft–Gault equation <60 ml/min] and old age (>70 years) were significant risk factors for grade 3/4 nonhematological adverse events by univariate analysis, whereas no significant risk factors were associated with hematological adverse events (Table 4). Multivariate analysis using binary logistic regression showed that renal impairment (Ccr <60 ml/min) was the only independent risk factor for grade 3–4 nonhematological adverse events (Table 4).
Associations between CYP2A6 polymorphisms and survival
The median follow-up time was 48.1 months (range, 22.7–69.3 months) in the W/W group, 45.0 months (17.1–91.0) in the W/V group, and 47.7 months (20.2–77.1) in the V/V group. A total of nine, seven, and one patients died in the W/V, V/V, and W/W groups, respectively. In all patients, the 3-year RFS and OS rates were 83.1 % (95 % CI, 77.7–88.5 %) and 94.8 % (95 % CI, 91.6–98.0 %), respectively.
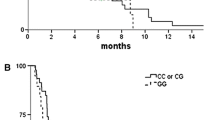
RFS significantly differed according to the CYP2A6 genotype. The 3-year RFS rates were 95.9 % (95 % CI, 90.4–100 %) in the W/W group, 83.1 % (95 % CI, 75.3–90.9 %) in the W/V group, and 72.5 % (95 % CI, 60.5–84.5 %) in the V/V group [p = 0.032; W/W vs. W/V or V/V = 95.9 % (95 % CI, 90.4–100 %) vs. 79.1 % (95 % CI, 72.4–85.8 %); p = 0.015] (Fig. 1a). After adjusting for pathological stage in the multivariate analysis, the CYP2A6 genotype remained a significant factor that affected RFS (Table 5). Carriers of W/V and V/V genotypes had a poorer RFS with a hazard ratio of 3.41 (95 % CI, 1.01–11.52; p = 0.049) and 4.03 (95 % CI, 1.16–13.93; p = 0.028), respectively, compared with the W/W genotype.
In terms of the OS outcomes, no significant differences were detected between the CYP2A6 genotypic groups; the 3-year OS rates for patients with W/W, W/V, and V/V genotypes were 100 %, 94.5 %, and 90.7 %, respectively (p = 0.166) (Fig. 1b).
Discussion
Recently, two large randomized phase III trials demonstrated survival benefits from adjuvant chemotherapy after D2 surgery for stage II–III GC. In the Japanese ACTS-GC trial, administering adjuvant S-1 for 12 months resulted in a significant survival advantage compared with surgery alone for OS (5-year OS: 71.7 % vs. 61.1 %; HR = 0.669; 95 % CI, 0.540–0.828) and RFS (5-year RFS: 65.4 % vs. 53.1 %; HR = 0.653; 95 % CI, 0.537–0.793) [5]. The Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) trial, which was performed in South Korea, China, and Taiwan, also showed a significant improvement in OS (5-year OS: 78 % vs. 69 %; HR = 0.66; 95 % CI, 0.51–0.85; p = 0.0015) and disease-free survival (DFS) (5-year DFS: 68 % vs. 53 %; HR = 0.58; 95 % CI, 0.47–0.72; p < 0.0001) with adjuvant capecitabine/oxaliplatin for 6 months compared with surgery alone [3, 4]. Although these two fluoropyrimidine-based regimens have been established as standard adjuvant chemotherapies, it remains unclear which regimen is better for an entire patient group with stage II–III disease or for a certain specific subset of patients. Studies of predictive biomarkers to guide adjuvant chemotherapy regimen are critical to maximize the benefits of adjuvant therapy.
In contrast to capecitabine, the metabolism of S-1 to 5-FU depends on the enzymatic activity of CYP2A6, which can be influenced by genetic polymorphisms [12–14, 22]. Therefore, CYP2A6 polymorphisms might affect the treatment outcomes of S-1-based chemotherapy, but not of capecitabine-based chemotherapy. Indeed, our present study findings indicate that CYP2A6 polymorphisms are correlated with the treatment efficacy of adjuvant S-1 chemotherapy after curative resection. The RFS was significantly better in patients with a W/W genotype than in patients with W/V or V/V genotypes. Patients with variant alleles that abolish or reduce enzyme activity or expression (W/V or V/V) had a 3.65-fold-higher HR of progression compared with patients with a wild-type (W/W) genotype (p = 0.032). These data are consistent with previous findings that showed an association between CYP2A6 genotypes and treatment efficacy in patients treated with S-1-based chemotherapy in a metastatic or perioperative setting [16, 23, 24]. A recent phase II study of perioperative S-1 plus docetaxel administered both pre- and postoperatively showed that patients with W/W or W/V genotypes had a better progression-free survival (PFS) (3-year PFS rate, 67.6 % vs. 33.3 %; p = 0.102) and OS rate (5-year OS rate, 75.6 % vs. 33.3 %; p = 0.032) compared with patients with a V/V genotype [23]. Similarly, patients treated with S-1 plus docetaxel for metastatic GC showed a different overall response rate (W/W vs. W/V vs. V/V = 79 % vs. 65 % vs. 30 %, respectively; p = 0.04) and PFS (W/W vs. W/V vs. V/V = 8.1 vs. 6.9 vs. 3.1 months, respectively; p = 0.0009) according to the CYP2A6 genotype [16].
Associations between CYP2A6 polymorphisms and the treatment outcomes of S-1 were further confirmed in a previous randomized phase II study that compared S-1 to capecitabine in patients with metastatic GC [24]. In the S-1 arm of that study, patients with W/W or W/V genotypes showed a longer median time to progression (4.1 vs. 2.3 months; p = 0.062) and OS (11.5 vs. 6.5 months; p = 0.034) compared to V/V patients. However, in the capecitabine arm of that study, patients with W/W or W/V genotypes showed a similar median time to progression (TTP) (3.3 vs. 3.6 months; p = 0.257) and OS (10.2 vs. 11.6 months; p = 0.756) compared with the V/V genotype cases. The poor treatment outcomes for S-1 in patients with CYP2A6 variant alleles suggest that these patients should be treated with either 5-FU or capecitabine, which do not require CYP2A6 activation, instead of S-1. Further studies are warranted to determine whether CYP2A6 genotypes guide treatment choices between adjuvant S-1 and capecitabine/oxaliplatin in patients with resected GC.
Regarding toxicity, we could not detect any association between CYP2A6 polymorphisms and adverse events in our present analysis. This finding is consistent with the results of previous studies in which adverse events did not differ according to CYP2A6 genotype during either the initial one to two cycles or all treatment cycles in an S-1 monotherapy or S-1-based combination therapy setting [14, 22]. However, we did detect an association between Ccr levels and adverse events related to S-1. Patients with Ccr levels <60 ml/min exhibited a higher incidence of grade ≥3 nonhematological toxicities compared with patients with Ccr levels ≥60 ml/min (55.0 % vs. 16.7 %; p = 0.001) (Table 5). This finding is also consistent with those of previous reports [16, 25]. As a component of S-1, CDHP is a potent inhibitor of dihydropyrimidine dehydrogenase, which is responsible for the rapid catabolism of 5-FU. Additionally, as more than 50 % of CDHP is excreted in urine, renal dysfunction can cause high exposure to CDHP and 5-FU [13]. Aoyama and colleagues reported that levels of Ccr <60 ml/min were a significant risk factor for the inability to continue adjuvant S-1 for 6 months, mostly because of adverse events; the continuation rate of S-1 at 6 months was 72.9 % in patients with Ccr ≥60 ml/min versus 40.0 % in those with Ccr <60 ml/min [25]. Patients with renal dysfunction should be carefully monitored for adverse events and be considered for S-1 dose adjustment.
The current study has some limitations. First, the pharmacokinetic data of S-1 were not collected in this study. Thus, we could not evaluate the association between CYP2A6 genotypes and pharmacokinetic profiles of S-1. Second, we grouped CYP2A6 polymorphisms into two groups (W/W vs. W/V and V/V). The grouping of CYP2A6 genotypes differed among studies. Although one study showed a trend of decreasing plasma concentrations of 5-FU in relationship to an increasing number of variant alleles (W/W vs. W/V vs. V/V) [23], several studies, including that study, reported statistically significant differences in the plasma concentration of 5-FU between patients with homozygous wild-type (W/W) versus patients with variant alleles (W/V or V/V) [14, 15, 23]. These inconsistent results might be caused at least in part by different CYP2A6 enzyme activity among variant alleles (*4, *7, *9, *10); the CYP2A6*4 allele causes a CYP2A6 gene deletion, which lacks activity, whereas CYP2A6*7, *9, and *10 cause decreased enzymatic activity of different degrees. Based on the foregoing data, we think that the grouping of CYP2A6 genotypes in this study might be justified.
In conclusion, CYP2A6 polymorphisms correlate with the treatment efficacy of S-1 adjuvant in patients with curatively resected GC. Patients with a W/W genotype are more likely to exhibit a good RFS compared with those harboring a W/V or V/V genotype. Large-scale prospective studies are warranted to validate these findings, which could provide useful information for selecting the best adjuvant chemotherapy between two current standard regimens for GC, that is, S-1 vs. capecitabine plus oxaliplatin.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–41.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Jeong JH, Ryu MH, Ryoo BY, Lee SS, Park I, Lee SH, et al. Safety and feasibility of adjuvant chemotherapy with S-1 for Korean patients with curatively resected advanced gastric cancer. Cancer Chemother Pharmacol. 2012;70:523–9.
Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, et al. Safety and feasibility of S-1 adjuvant chemotherapy for gastric cancer in elderly patients. Gastric Cancer. 2012;15:76–82.
Jo JC, Baek JH, Koh SJ, Kim H, Min YJ, Lee BU, et al. Adjuvant chemotherapy for elderly patients (aged 70 or older) with gastric cancer after a gastrectomy with D2 dissection: a single center experience in Korea. Asia Pac J Clin Oncol. 2015;11:282–7
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Ikeda K, Yoshisue K, Matsushima E, Nagayama S, Kobayashi K, Tyson CA, et al. Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res. 2000;6:4409–15.
Kaida Y, Inui N, Suda T, Nakamura H, Watanabe H, Chida K. The CYP2A6*4 allele is determinant of S-1 pharmacokinetics in Japanese patients with non-small-cell lung cancer. Clin Pharmacol Ther. 2008;83:589–94.
Fujita K, Yamamoto W, Endo S, Endo H, Nagashima F, Ichikawa W, et al. CYP2A6 and the plasma level of 5-chloro-2,4-dihydroxypyridine are determinants of the pharmacokinetic variability of tegafur and 5-fluorouracil, respectively, in Japanese patients with cancer given S-1. Cancer Sci. 2008;99:1049–54.
Kim KP, Jang G, Hong YS, Lim HS, Bae KS, Kim HS, et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br J Cancer. 2011;104:605–12.
Park SR, Hong YS, Lim H-S, Seong M-W, Kong S-Y, Kim SY, et al. Phase I clinical and pharmacokinetic/pharmacogenetic study of a triplet regimen of S-1/irinotecan/oxaliplatin in patients with metastatic colorectal or gastric cancer. Cancer Chemother Pharmacol. 2013;72:953–64.
Kong S-Y, Lim H-S, Nam B-H, Kook M-C, Kim Y-W, Ryu KW, et al. Association of CYP2A6 polymorphisms with S-1 plus docetaxel therapy outcomes in metastatic gastric cancer. Pharmacogenomics. 2009;10:1147–55.
Kwon JT, Nakajima M, Chai S, Yom YK, Kim HK, Yamazaki H, et al. Nicotine metabolism and CYP2A6 allele frequencies in Koreans. Pharmacogenetics. 2001;11:317–23.
Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–26.
Mwenifumbo JC, Myers MG, Wall TL, Lin S-K, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenet Genomics. 2005;15:189–92.
Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–97.
Xu C, Rao YS, Xu B, Hoffmann E, Jones J, Sellers EM, et al. An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles. Biochem Biophys Res Commun. 2002;290:318–24.
Park SR, Kong SY, Nam BH, Choi IJ, Kim CG, Lee JY, et al. CYP2A6 and ERCC1 polymorphisms correlate with efficacy of S-1 plus cisplatin in metastatic gastric cancer patients. Br J Cancer. 2011;104:1126–34.
Kim YW, Kim MJ, Ryu KW, Lim HS, Lee JH, Kong SY, et al. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer 2015. doi:10.1007/s10120-015-0490-3
Park SR, Kong S-Y, Kim M-J, Kim MK, Nam B-H, Choi M, et al (2013) A randomized phase II study of S-1 versus capecitabine as first-line chemotherapy in the elderly metastatic gastric cancer patients with/without poor performance status: clinical and pharmacogenetic results. AACR Annu Meet 73:abstr LB–172
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013;16:133–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Funding
This work was not supported by any specific sources of direct or indirect funding.
Conflict of interest
Y.K.K. is a consultant for TAIHO.
Additional information
J.H. Jeong and S.R. Park contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jeong, J.H., Park, S.R., Ahn, Y. et al. Associations between CYP2A6 polymorphisms and outcomes of adjuvant S-1 chemotherapy in patients with curatively resected gastric cancer. Gastric Cancer 20, 146–155 (2017). https://doi.org/10.1007/s10120-015-0586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0586-9