Abstract
Epoxidation of the carbon-carbon double bonds on unsaturated rubber macromolecules can produce novel modified rubber species with special properties, and construct eco-friendly crosslinking pathway via the reaction of epoxide groups to solve the problems brought by conventional sulfur vulcanization system. In this contribution, a novel modified product of isobutylene isoprene rubber (IIR), epoxy-functionalized IIR (EIIR) was successfully prepared by in situ epoxidation technique for the first time, and the crosslinking of EIIR was achieved by the reaction of oxirane groups with maleic anhydride (MAH) without additional additives. The reaction conditions for preparing EIIR were optimized through systematic research on the epoxidation process. Under optimal condition, the degree of epoxidation of the rubber reached around 99% without side reactions. The obtained EIIR/carbon black composites cured by MAH had excellent mechanical properties comparable to those of IIR composites. More importantly, compared with IIR composites, the air-tightness of the EIIR composites was improved by about 50%, and the flexural fatigue life of first-level cracks and sixth-level cracks was increased by several times. The significant improvement of these properties is of great significance for the application safety and energy saving of IIR materials.
Similar content being viewed by others
REFERENCES
Coran, A. Y. Vulcanization. Sci. Technol. Rubber. Academic Press. 1994, DOI: https://doi.org/10.1016/b978-0-08-051667-7.50012-3
Roy, K.; Alam, M. N.; Mandal, S. K.; Debnath, S. C. Sol-gel derived nano zinc oxide for the reduction of zinc oxide level in natural rubber compounds. J. Sol-Gel Sci. Technol. 2014, 70, 378–384.
Mostoni, S.; Milana, P.; Credico, B. D.; D’Arienzo, M.; Scotti, R. Zinc-based curing activators: new trends for reducing zinc content in rubber vulcanization process. Catalysts. 2019, 9, 664.
Lin, T. F.; Zhang, X. H.; Tang, Z. H.; Guo, B. C. Renewable conjugated acids as curatives for high-performance rubber/silica composites. Green Chem. 2015, 17, 3301–3305.
Hansson, C. Allergic contact dermatitis from (N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine and from compounds in polymerized 2,2,4-trimethyl-1, 2-dihydroquinoline. Contact Dermatitis. 1994, 30, 114–115.
Kroft, E. B. M.; Van Der Valk, P. G. M. Occupational contact dermatitis of both hands because of sensitization of black rubber. Contact Dermatitis. 2008, 58, 125–126.
Yamano, T.; Shimizu, M. Skin sensitization potency and cross-reactivity of p-phenylenediamine and its derivatives evaluated by non-radioactive murine local lymph node assay and guinea-pig maximization test. Contact Dermatitis. 2009, 60, 193–198.
Zhang, G. G.; Zhou, X. X.; Zhang, Q. L. Editorial corner-a personal view Current issues for rubber crosslinking and its future trends of green chemistry strategy. Express Polym. Lett. 2019, 13, 406–406.
Naskar, K. Thermoplastic elastomers based on PP/EPDM blends by dynamic vulcanization. Rubber Chem. Technol. 2007, 80, 504–519.
Zhang, X. H.; Tang, Z. H.; Guo, B. C. Regulation of mechanical properties of diene rubber cured by oxa-Michael Reaction via manipulating network structure. Polymer. 2018, 144, 57–64.
Zhang, G. G.; Feng, H.; Liang, K.; Wang, Z.; Li, X. L.; Zhou, X. X.; Guo, B. C.; Zhang, L. Q. Design of next-generation cross-linking structure for elastomers toward green process and a real recycling loop. Sci. Bull. 2020, 65, 889–898.
Feng, H. R.; Tian, C.; Zhang, G. G.; Zhang, L. Q. Catalyst-free curing and closed-loop recycling of carboxylated functionalized rubber by a green crosslinking strategy. Polymer 2021, 234, 124237–124247.
Pire, M.; Norvez, S.; Iliopoulos, I.; Rossignol, B. L.; Leibler, L. Epoxidized natural rubber/dicarboxylic acid self-vulcanized blends. Polymer 2010, 51, 5903–5909.
Zhang, G. G.; Zhou, X. X.; Liang, K.; Guo, B. C.; Li, X. L.; Wang, Z.; Zhang, L. Q. Mechanically robust and recyclable EPDM rubber composites by a green cross-linking strategy. ACS Sustainable Chem. Eng. 2019, 7, 11712–11720.
Pire, M.; Norvez, S.; Iliopoulos, I.; Rossignol, B. L.; Leibler, L. Imidazole-promoted acceleration of crosslinking in epoxidized natural rubber/dicarboxylic acid blends. Polymer 2011, 52, 5243–5249.
Pire, M.; Lorthioir, C.; Oikonomou, E. K.; Norvez, S.; Iliopoulos, I.; Rossignol, B. L.; Leibler, L. Imidazole-accelerated crosslinking of epoxidized natural rubber by dicarboxylic acids: a mechanistic investigation using NMR spectroscopy. Polym. Chem. 2012, 3, 946–953.
Pire, M.; Norvez, S.; Iliopoulos, I.; Rossignol, B. L.; Leibler, L. Dicarboxylic acids may compete with standard vulcanization processes for crosslinking epoxidised natural rubber. Compos. Interface 2014, 21, 45–50.
Algaily, B.; Kaewsakul, W.; Sarkawi, S. S.; Kalkornsurapranee, E. Enabling reprocessability of ENR-based vulcanisates by thermochemically exchangeable ester crosslinks. Plast. Rubber Compos. 2021, 50, 315–328.
Chilkoor, G.; Sarder, R.; Islam, J.; ArunKumar, K. E.; Ratnayake, I. Maleic anhydride-functionalized graphene nanofillers render epoxy coatings highly resistant to corrosion and microbial attack. Carbon. 2020, 159, 586–597.
Espana, J. M.; Sánchez-Nacher, L.; Boronat, T.; Fombuena, V.; Balart, R. Properties of biobased epoxy resins from epoxidized soybean oil (ESBO) cured with maleic anhydride (MA). J. Am. Oil Chem. Soc. 2012, 89, 2067–2075.
Kolář, F.; Svítilová, J. Kinetics and mechanism of curing epoxy/anhydride systems. Acta Geodyn. Geomater. 2007, 4, 85–92.
Srirachya, N.; Kobayashi, T.; Boonkerd, K. An alternative crosslinking of epoxidized natural rubber with maleic anhydride. Key Eng. Mater. 2017, 748, 84–90.
Zhang, G. G.; Liang, K.; Feng, H. R.; Pang, J. X.; Liu, N. Q.; Li, X. L. Design of epoxy-functionalized styrene-butadiene rubber with bio-based dicarboxylic acid as a cross-linker toward the green-curing process and recyclability. Ind. Eng. Chem. Res. 2020, 59, 10447–10456.
Yang, C. L.; Li, T.; Li, Z. P. Effect of heating on properties of ENR. China Rubber Ind. 2002, 49, 400–402.
Liu, Y. J.; Tang, Z. H.; Wu, S. W.; Guo, B. C. Integrating sacrificial bonds into dynamic covalent networks toward mechanically robust and malleable elastomers. ACS Macro Lett. 2019, 8, 193–199.
Tang, Z. H.; Liu, Y. J.; Guo, B. C.; Zhang, L. Q. Malleable, mechanically strong, and adaptive elastomers enabled by interfacial exchangeable bonds. Macromolecules. 2017, 50, 7584–7592.
Cao, L. M.; Fan, J. F.; Huang, J. R.; Chen, Y. K. A robust and stretchable cross-linked rubber network with recyclable and self-healable capabilities based on dynamic covalent bonds. J. Mater. Chem. A 2019, DOI: https://doi.org/10.1039/C8TA11587G
Zhu, Y.; Gao, J. L.; Zhang, L. J.; Peng, Y.; Wang, H.; Ling, F. W.; Huang, G. S.; Wu, J. R. An interfacial dynamic crosslinking approach toward catalyst-free and mechanically robust elastomeric vitrimer with a segregated structure. Chinese J. Polym. Sci. 2021, 39, 201–210.
Tanrattanakul, V.; Wattanathai, B.; Tiangjunya, A.; Muhamud, P. In situ epoxidized natural rubber: improved oil resistance of natural rubber. J. Appl. Polym. Sci. 2003, 90, 261–269.
Pu, L.; Zhang, R.; Zhu, Y. Development and optimization of epoxidation process for natural rubber. Yunnan Chem. Technol. 2021, 48, 46–49.
Li, C. B.; Wang, H.; Feng, Z.; Yang, S. T. Preparation and characterization of epoxidized natural rubber by in-situ method. New Chem. Mater. 2012, 40, 124–126+130.
Puskas, J. E.; Wilds, C. Kinetics of the epoxidation of butyl rubber; development of a high precision analytical method for unsaturation measurement. Rubber Chem. Technol. 1994, 67, 329–341.
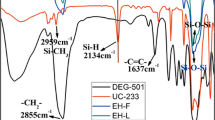
Wu, J.; Cui, B. C.; Wang, Y.; Shi, Y.; Ren, X. B.; Xu, H. D.; Liu, Z. X.; Hao, F. L.; Zhang, L. Q. Synthesis and characterization of epoxidized butyl rubber. Acta Polymerica Sinica (in Chinese) 2022, 53, 185–192.
Wang, Y. M.; Cao, R. W.; Wang, M. H.; Liu, X. M.; Zhao, X. Y.; Lu, Y. L.; Feng, A. C.; Zhang, L. Q. Design and synthesis of phenyl silicone rubber with functional epoxy groups through anionic copolymerization and subsequent epoxidation. Polymer 2020, 186, 122077–12084.
James, A. P.; Johnstone, R. A. W.; McCarron, M.; Sankey, J. P.; Trenbirth, B. 5-Hydroperoxycarbonylphthalimide: a new reagent for epoxidation. Chem. Commun. 1998, 429–430.
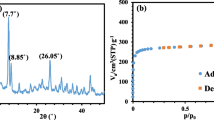
Zhang, X.; Niu, K. J.; Song, W. X.; Yan, S. K.; Zhao, X. Y.; Lu, Y. L.; Zhang, L. Q. The effect of epoxidation on strain-induced crystallization of epoxidized natural rubber. Macromol. Rapid Commun. 2019, 40, 1900042–1900046.
Roy, S.; Gupta, B. R.; Maiti, B. R. Effect of acid concentration and other reaction parameters on epoxidation of natural rubber latex. Ind. Eng. Chem. Res. 1991, 30, 2573–2576.
Jacobi, M. M.; Santin, C. K.; Alegre, M.; Schuster, R. H. Study of the epoxidation of polydiene rubbers I. Influence of the microstructure on the epoxidation of SBR with performic acid. KGK-Kautsch. Gummi. Kunstst. 2002, 55, 590–595.
Chu, C. Y.; Vukov, R. Determination of the structure of butyl rubber by NMR spectroscopy. Macromolecules 1985, 18, 1423–1430.
Jacobi, M. M.; Schneider, L. K.; Freitas, L. L.; Schuster, R. H. Properties and morphology of thermoplastic vulcanizates based on PP/SBR and PP/EpSBR. KGK-Kautsch. Gummi. Kunstst. 2006, 59, 49–54.
Johnson, T.; Thomas, S. Nitrogen/oxygen permeability of natural rubber, epoxidised natural rubber and natural rubber/epoxidised natural rubber blends. Polymer 1999, 40, 3223–3228.
Li, Z. P.; Lan, J. Effects of epoxidation of natural rubber on its properties. Chinese J. Appl. Chem. 1996, 13, 49–51.
Zhao, X. Y.; Niu, K. J.; Xu, Y.; Peng, Z.; Jia, L.; Hui, D.; Zhang, L. Q. Morphology and performance of NR/NBR/ENR ternary rubber composites. Compos. PartB-Eng. 2016, 107, 106–112.
Wang, Y. Y.; Shi, M. X.; Sun, Y.; Tie, W. Y.; Zhang, L. S. Production technology and market analysis of butyl rubber. China Elastomerics 2010, 20, 80–84.
Grubisic, V. Air tightness control of passenger car wheels. Engineering 2017, 9, 171–180.
Roychoudhury, A.; De, P. P. Reinforcement of epoxidized natural rubber by carbon black: effect of surface oxidation of carbon black particles. J. Appl. Polym. Sci. 1993, 50, 181–186.
Wang, M. J.; Wolff, S.; Donnet, J. B. Filler-elastomer interactions. Part III. Carbon-black-surface energies and interactions with elastomer analogs. Rubber Chem. Technol. 1991, 64, 714–736.
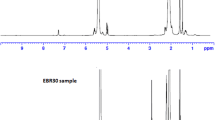
Bradbury, J. H.; Perera, M. C. S. Epoxidation of natural rubber studied by NMR spectroscopy. J. Appl. Polym. Sci. 1985, 30, 3347–3364.
Hamzah, R.; Bakar, M. A.; Khairuddean, M.; Mohammed, I. A.; Adnan, R. A structural study of epoxidized natural rubber (ENR-50) and its cyclic dithiocarbonate derivative using NMR spectroscopy techniques. Molecules 2012, 17, 10974–10993.
Gan, S. N.; Hamid, Z. A. Partial conversion of epoxide groups to diols in epoxidized natural rubber. Polymer 1997, 38, 1953–1956.
Dahham, O. S.; Hamzah, R.; Bakar, M. A.; Zulkepli, N. N.; Dahham, S. S.; Ting, S. S. NMR study of ring opening reaction of epoxidized natural rubber in presence of potassium hydroxide/isopropanol solution. Polym. Test. 2017, 59, 55–66.
Vernekar, S. P.; Sabne, M. B.; Patil, S. D.; Patil, A. S.; Idage, S. B.; Avadhani, C. V.; Sivaram, S. Effect of latex concentration on epoxidation of natural rubber (NR) latex. J. Appl. Polym. Sci. 1992, 44, 2107–2114.
Hashim, A. S.; Kohjiya, S. Preparation and properties of epoxidized natural rubber network crosslinked by ring opening reaction. Polym. Gels Networks 1994, 2, 219–227.
Huang, C.; Ge, Z.; Zhao, B. B.; Wang, Z.; Luo, Y. J. Effects of DMP-30 on curing behavior of epoxy resin/maleicanhydride systems. J. Chem. Eng. Chinese Universities 2017, 31, 197–204.
Papirer, E.; Dentzer, J.; Li, S.; Donnet, J. B. Surface groups on nitric acid oxidized carbon black samples determined by chemical and thermodesorption analyses. Carbon 1991, 29, 69–72.
Manna, A. K.; De, P. P.; Tripathy, D. K.; De, S. K. Chemical interaction between surface oxidized carbon black and epoxidized natural rubber. Rubber Chem. Technol. 1997, 70, 624–633.
Menard, K. P.; Bilyeu, B. W., in Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, John Wiley & Sons Ltd, New York, 2006, p. 16.
Tang, Z. H.; Wu, X. H.; Guo, B. C.; Zhang, L. Q.; Jia, D. Preparation of butadiene-styrene-vinyl pyridine rubber-graphene oxide hybrids through co-coagulation process and in situ interface tailoring. Mater. Chem. 2012, 22, 7492–7501.
Qiao, H.; Wang, R. G.; Yao, H.; Zhou, X. X.; Lei, W. W.; Hu, X. R.; Zhang, L. Q. Preparation of graphene oxide/bio-based elastomer nanocomposites through polymer design and interface tailoring. Polym. Chem. 2015, 6, 6140–6151.
Zheng, L.; Jerrams, S.; Su, T.; Xu, Z. C.; Zhang, L. Q.; Liu, L.; Wen, S.P. Enhanced covalent interface, crosslinked network and gas barrier property of functionalized graphene oxide/styrene-butadiene rubber composites triggered by thiol-ene click reaction. Compos. Part B-Eng. 2020, 197, 108186.
Liu, W.; Lv, L. T.; Yang, Z. L.; Zheng, Y. Q.; Wang, H. The effect of OMMT on the properties of vehicle damping carbon black-natural rubber composites. Polymers 2020, 12, 1983–1993.
Huneau, B.; Masquelier, I.; Marco, Y.; Saux, V. L.; Noizet, S.; Schiel, C.; Charrier, P. Fatigue crack initiation in a carbon black-filled natural rubber. Rubber Chem. Technol. 2016, 89, 126–141.
Dong, B.; Zhang, L. Q.; Wu, Y. P. Influences of different dimensional carbon-based nanofillers on fracture and fatigue resistance of natural rubber composites. Polym. Test. 2017, 63, 281–288.
Ishak, Z. A. M.; Bakar, A. A.; Ishiaku, U. S.; Hashim, A. S.; Azahari, B. An investigation of the potential of rice husk ash as a filler for epoxidized natural rubber—II. Fatigue behaviour. Eur. Polym. J. 1997, 33, 73–79.
Ismail, H.; Jaffri, R. M.; Rozman, H. D. Oil palm wood flour filled natural rubber composites: fatigue and hysteresis behaviour. Polym. Int. 2000, 49, 618–622.
Sun, Y. N.; Cheng, P. F.; Cui, B. P.; Sun, X. H. Effects of CB and curing degree on fatigue properties of NR vulcanizates. China Elastomerics 2017, 27, 34–38.
Wang, H.; Wei, Y. T.; Wang, J. Influencing factors and research methods of rubber material fatigue life. China Rubber Ind. 2020, 67, 723–735.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China (Nos. 2022YFB3704800, 2022YFB3704802 and 52273051) and the Fundamental Research Funds for the Central Universities (No. JD2221).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no interest conflict.
Electronic Supplementary Information
Rights and permissions
About this article
Cite this article
Gao, H., Cui, BC., Zheng, HB. et al. Epoxidation Functionalized Isobutylene Isoprene Rubber toward Green-curing Pathway and High-performance Composites. Chin J Polym Sci 41, 1818–1828 (2023). https://doi.org/10.1007/s10118-023-2986-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-023-2986-3