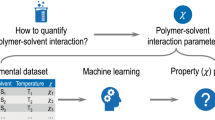
Abstract
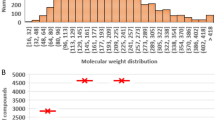
Polymer-solvent interaction is a fundamentally important concept routinely described by the Flory-Huggins interaction (χ), Hildebrand solubility(✉δ) and the relative energy difference (RED) determined from Hansen solubility in experimental, theoretical and simulation studies. Here we performed a machine learning study based on a comprehensive and representative dataset covering the interaction pairs from 81 polymers and 1221 solvents. The regression models provide the coefficients of determination in the range of 0.86–0.94 and the classification models deliver the area under the receiver operating characteristic curve (AUCs) better than 0.93. These models were integrated into a newly developed software polySML-PSI. Important features including LogP, molar volume and dipole are identified, and their non-linear, nonmonotonic contributions to polymer-solvent interactions are presented. The widely known “like-dissolve-like” rule and two broadly used empirical equations to estimate χ as a function of temperature or Hansen solubility are also evaluated, and the polymer-specified constants are presented. This study provides a quantitative reference and a tool to understand and utilize the concept of polymer-solvent interactions.
Similar content being viewed by others
References
Flory, P. J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61.
Flory, P. J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1941, 9, 660–660.
Huggins, M. L. Some properties of solutions of long-chain compounds. J. Phys. Chem. 1942, 46, 151–158.
Huggins, M. L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718.
Blanks, R. F.; Prausnitz, J. Thermodynamics of polymer solubility in polar and nonpolar systems. Ind. Eng. Chem. Fundam. 1964, 3, 1–8.
Bae, Y. H.; Okano, T.; Kim, S. W. Temperature dependence of swelling of crosslinked poly(N, N′-alkyl substituted acrylamides) in water. J. Polym. Sci., Part B: Polym. Phys. 1990, 28, 923–936.
Schweizer, K. S.; Yethiraj, A. Polymer reference interaction site model theory: new molecular closures for phase separating fluids and alloys. J. Chem. Phys. 1993, 98, 9053–9079.
Zhuang, B.; Ramanauskaite, G.; Koa, Z. Y.; Wang, Z. G. Like dissolves like: a first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275.
Geoghegan, M.; Krausch, G. Wetting at polymer surfaces and interfaces. Prog. Polym. Sci. 2003, 28, 261–302.
Chen, J.; Zhuang, H.; Zhao, J.; Gardella, J. A. Solvent effects on polymer surface structure. Surf. Interface Anal. 2001, 31, 713–720.
Guillen, G. R.; Pan, Y.; Li, M.; Hoek, E. M. V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: a review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817.
König-Mattern, L.; Linke, S.; Rihko-Struckmann, L.; Sundmacher, K. Computer-aided solvent screening for the fractionation of wet microalgae biomass. Green Chem. 2021, 23, 10014–10029.
Xia, J.; Guo, H.; Travesset, A. On the thermodynamic stability of binary superlattices of polystyrene-functionalized nanocrystals. Macromolecules 2020, 53, 9929–9942.
Chen, J.; Zha, L.; Hu, W. Effect of solvent selectivity on crystallization-driven fibril growth kinetics of diblock copolymers. Polymer 2018, 138, 359–362.
Tao, Y.; Olsen, B. D.; Ganesan, V.; Segalman, R. A. Domain size control in self-assembling rod-coil block copolymer and homopolymer blends. Macromolecules 2007, 40, 3320–3327.
Danquah, M.; Fujiwara, T.; Mahato, R. I. Self-assembling methoxypoly(ethylene glycol)-b-poly(carbonate-co-L-lactide) block copolymers for drug delivery. Biomaterials 2010, 31, 2358–2370.
Jimenez, J.; Ford, E. Mapping wet vs gel spinning in Hansen space. Polymer 2021, 230, 124079.
Robinson, J. Solvent flux through dense polymeric nanofiltration membranes. J. Membr. Sci. 2004, 230, 29–37.
Orwoll, R. A.; Arnold, P. A., Polymer-solvent interaction parameter χ. In Physical properties of polymers handbook, Mark, J. E., Ed. Springer: 2007; pp. 233–257.
Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical assessment of the hildebrand and hansen solubility parameters for polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194.
Hansen, C. Hansen Solubility Parameters: A User’s Handbook. CRC Press: 2007, p. 546.
Khansary, M. A. Vapor pressure and Flory-Huggins interaction parameters in binary polymeric solutions. Korean J. Chem. Eng. 2016, 33, 1402–1407.
Schwahn, D.; Willner, L. Phase Behavior and Flory- Huggins interaction parameter of binary polybutadiene copolymer mixtures with different vinyl content and molar volume. Macromolecules 2002, 35, 239–247.
Sun, Z.; Wang, C. H. Determination of Flory-Huggins interaction parameter and self-diffusion coefficients in ternary polymer solutions by quasielastic light scattering. J. Chem. Phys. 1995, 103, 3762–3766.
DiPaola-Baranyi, G.; Guillet, J. Estimation of polymer solubility parameters by gas chromatography. Macromolecules 1978, 11, 228–235.
Deshpande, D.; Tyagi, O. Gas chromatographic behavior of poly(vinyl acetate) at temperatures encompassing Tg: determination of Tg and η. Macromolecules 1978, 11, 746–751.
Emerson, J. A.; Toolan, D. T. W.; Howse, J. R.; Furst, E. M.; Epps, T. H. Determination of solvent-polymer and polymer-polymer Flory-Huggins interaction parameters for poly(3-hexylthiophene) via solvent vapor swelling. Macromolecules 2013, 46, 6533–6540.
Errede, L. Polymer swelling. 5. Correlation of relative swelling of poly(styrene-co-divinylbenzene) with the Hildebrand solubility parameter of the swelling liquid. Macromolecules 1986, 19, 1522–1525.
Sweere, A. J.; Fraaije, J. G. Prediction of polymer-solvent miscibility properties using the force field based quasi-chemical method PAC-MAC. Polymer 2016, 107, 147–153.
Cui, F.; Chen, W.; Kong, X.; Liu, L.; Shi, C.; Li, Y. Anomalous dynamics of water in polyamide matrix. J. Phys. Chem. B 2019, 123, 3086–3095.
Chen, W.; Cui, F.; Liu, L.; Li, Y. Assembled structures of perfluorosulfonic acid ionomers investigated by anisotropic modeling and simulations. J. Phys. Chem. B 2017, 121, 9718–9724.
Lei, Q.-L.; Ciamarra, M. P.; Ni, R. Nonequilibrium strongly hyperuniform fluids of circle active particles with large local density fluctuations. Sci. Adv. 2019, 5, eaau7423.
Li, Y.; Liu, L.; Chen, W.; An, L. Materials genome: research progress, challenges and outlook. Sci. Sin. Chim. 2018, 48, 243–255.
Liu, L.; Ding, F.; Li, Y. Big data approach on polymer materials: fundamental, progress and challenge. Acta Polymerica Sinica (in Chinese) 2022, 52, 1–17.
Chandrasekaran, A.; Kim, C.; Venkatram, S.; Ramprasad, R. A deep learning solvent-selection paradigm powered by a massive solvent/nonsolvent database for polymers. Macromolecules 2020, 53, 4764–4769.
Greaves, T. L.; Drummond, C. J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120.
Postel, S.; Schneider, C.; Wessling, M. Solvent dependent solute solubility governs retention in silicone based organic solvent nanofiltration. J. Membr. Sci. 2016, 497, 47–54.
Sanchez-Lengeling, B.; Roch, L. M.; Perea, J. D.; Langner, S.; Brabec, C. J.; Aspuru-Guzik, A. A Bayesian approach to predict solubility parameters. Adv. Theory Simul. 2018, 2, 1800069.
Mark, J. E. Physical properties of polymer handbook. Springer: 2007, Vol. 1076.
Potter, C. B.; Davis, M. T.; Albadarin, A. B.; Walker, G. M. Investigation of the dependence of the Flory-Huggins interaction parameter on temperature and composition in a drug-polymer system. Mol. Pharm. 2018, 15, 5327–5335.
Van Dijk, M.; Wakker, A. Some observations on the behaviour of the thermodynamic interaction parameter in dilute polymer solutions. Polymer 1993, 34, 132–137.
Lindvig, T.; Michelsen, M. L.; Kontogeorgis, G. M. A Flory-Huggins model based on the Hansen solubility parameters. Fluid Phase Equilib. 2002, 203, 247–260.
Bicerano, J. Prediction of Polymer Properties. CRC Press: 2002.
Barton, A. F. M. CRC handbook of solubility parameters and other cohesion parameters, second edition. CRC Press Taylor and Francis: 2017 p. 1–739.
Zade, S. S.; Zamoshchik, N.; Bendikov, M. From short conjugated oligomers to conjugated polymers. Lessons from studies on long conjugated oligomers. Acc. Chem. Res. 2011, 44, 14–24.
RDKit: open-source cheminformatics. http://www.rdkit.org/. (accessed 23 Nov).
Rappé, A. K.; Casewit, C. J.; Colwell, K.; Goddard III, W. A.; Skiff, W. M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035.
Stewart, J. J. P. MOPAC2016. http://OpenMOPAC.net (accessed 29 Nov).
Bajusz, D.; Rácz, A.; Héberger, K. Why is Tanimoto index an appropriate choice for fingerprint-based similarity calculations. J. Cheminform. 2015, 7, 1–13.
Liu, L.; Chen, W.; Liu, T.; Kong, X.; Zheng, J.; Li, Y. Rational design of hydrocarbon-based sulfonated copolymers for proton exchange membranes. J. Mater. Chem. A 2019, 7, 11847–11857.
Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. Lightgbm: a highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 2017, 30, 3146–3154.
Liu, T.; Liu, L.; Cui, F.; Ding, F.; Zhang, Q.; Li, Y. Predicting the performance of polyvinylidene fluoride, polyethersulfone and polysulfone filtration membranes using machine learning. J. Mater. Chem. A 2020, 8, 21862–21871.
Spowage, B. M.; Bruce, C. L.; Hirst, J. D. Interpretable correlation descriptors for quantitative structure-activity relationships. J. Cheminform. 2009, 1, 1–13.
Shang, B. Z.; Wang, Z.; Larson, R. G. Effect of headgroup size, charge, and solvent structure on polymer—Micelle interactions, studied by molecular dynamics simulations. J. Phys. Chem. B 2009, 113, 15170–15180.
Ivanova, A.; Madjarova, G.; Tadjer, A.; Gospodinova, N. Effect of solvation and intermolecular interactions on the structure and optical properties of PANI oligomers. Int. J. Quantum Chem. 2006, 106, 1383–1395.
Box, K.; Comer, J. Using measured pKa, LogP and solubility to investigate supersaturation and predict BCS class. Curr. Drug Metab. 2008, 9, 869–878.
Papanu, J.; Hess, D.; Soane, D.; Bell, A. Dissolution of thin poly(methyl methacrylate) films in ketones, binary ketone/alcohol mixtures, and hydroxy ketones. J. Electrochem. Soc. 1989, 136, 3077.
Zhao, Y.; Liu, W.; Pei, X.; Yao, D. Refinement of the theoretical solubility model and prediction of solute solubility in mixed solvent systems. Fluid Phase Equilib. 2017, 437, 43–55.
Song, Z.; Chiang, S. W.; Chu, X.; Du, H.; Li, J.; Gan, L.; Xu, C.; Yao, Y.; He, Y.; Li, B. Effects of solvent on structures and properties of electrospun poly(ethylene oxide) nanofibers. J. Appl. Polym. Sci. 2018, 135, 45787.
Etxabarren, C.; Iriarte, M.; Uriarte, C.; Etxeberria, A.; Iruin, J. Polymer-solvent interaction parameters in polymer solutions at high polymer concentrations. J. Chromatogr. A 2002, 969, 245–254.
Chang, T. Polymer characterization by interaction chromatography. J. Polym. Sci., Part B: Polym. Phys. 2005, 43, 1591–1607.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (Nos. 21774128, U1832177, 22173094, 51988102), CAS Key Research Program of Frontier Sciences (No. QYZDY-SSW-SLH027). We are grateful to Network and Computing Center, Changchun Institute of Applied Chemistry for essential support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Notes
The authors declare no competing financial interest.
Electronic Supplementary Information
Rights and permissions
About this article
Cite this article
Liu, TL., Liu, LY., Ding, F. et al. A Machine Learning Study of Polymer-Solvent Interactions. Chin J Polym Sci 40, 834–842 (2022). https://doi.org/10.1007/s10118-022-2716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-022-2716-2