Abstract
Predicting the spread of invasive species and understanding the role of niche dynamics in niche transferability are critical challenges in the management of biological invasions, both theoretically and practically. We used complementary species distribution modelling approaches, such as multivariate niche analysis and reciprocal distribution models, to test the niche conservatism hypothesis and to predict the potential distribution of the fishhook waterflea, Cercopagis pengoi. Our analysis indicated a significant similarity between its native and invasive ranges, suggesting that a subset of the Ponto-Caspian propagules may have been the founders of European populations. However, our results contradict the niche conservatism hypothesis, showing that C. pengoi has not fully occupied the available niche within its current invasive ranges. Moreover, we observed a notable niche expansion, reflecting a significant shift in niche following its intercontinental introduction in North America. Given the suitability of new environments for the expansion of C. pengoi and its tendency to evade detection prior to population surges, we recommend a focus on early detection through monitoring of both water columns and bottom sediments. This should be complemented by strict enforcement of ballast water regulations to curtail its spread in North America, Europe, and other suitable non-native regions globally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic invasions can affect biodiversity and ecosystem services at different levels by outcompeting native species, disruptin trophic relationships, and—among others—altering nutrient dynamics (Havel et al. 2015; Bellard et al. 2016; Cuthbert et al. 2021). The extent of invasive non-native species introductions and the severity of their impacts depend on the connectivity of the aquatic system, the frequency and magnitude of novel introductions (i.e. propagule pressure and colonisation pressure; Lockwood et al. 2005; Briski et al. 2012), and the taxon considered, as well as the effectiveness of the consequently applied management actions (Ahmed et al. 2022). Likewise, distinguishing temporal and spatial dynamics within the invasion process is essential for informing decision-making, optimising current and future conservation interventions, as well as minimising the impacts of invasive species (Ricciardi 2007; Hulme 2009; Haubrock et al. 2022). A common method to estimate the potential spread of an invasive non-native species involves evaluating its niches in environmental and geographic contexts using species distribution modelling (SDM). These models can correlate species occurrences from the native and invaded ranges with environmental data to predict the environmental suitability and invasion potential in space and time (Soberón and Nakamura 2009; Araújo and Peterson 2012). Nevertheless, climatic modelling assumptions oversimplify the relationships between species and their environment and whether these relationships are affected by e.g. (i) genetic and phenotypic variations, (ii) dispersal features, (iii) biotic interactions and (iv) niche conservatism (Liu et al. 2022; Han et al. 2023). The niche conservatism hypothesis—a fundamental assumption on which SDM-based predictions have been developed—states that the niches of species remain conserved across different spatial and temporal scales (Soberón and Nakamura 2009; Wiens et al. 2010). Approaches for quantifying niche dynamics have also been developed to study the success of non-native invasive species in new environments (see a review of 434 species at Liu et al. 2020). While invasive non-native species often maintain their niches (Liu et al. 2022), niche evolution is a significant aspect of their invasiveness (Hui et al. 2023). This evolution can result from environmental selection in new ranges (Card et al. 2018), changes in ecological interactions like predation pressure (Tingley et al. 2014), and adaptations in morphology, physiology, and function (Macêdo et al. 2021). Understanding whether invasive non-native species retain their niches after successful establishments is crucial for more precise modelling of potential range expansions and for guiding effective management strategies.
Ecological and evolutionary changes, notably including biological invasions, are significantly altering and threatening biodiversity globally (Mooney and Cleland 2001). This highlights the need to investigate variations in landscapes affected by invasions and their diverse impacts on native species and their communities. In our study, we used SDMs to explore the ecological and biogeographical characteristics of Cercopagis pengoi Ostroumov, 1891, a small invertebrate with an adult body size of 1–3 mm that is listed as one of the ‘100 of the World’s Worst Invasive Alien Species’ (Luque et al. 2014). Native to the Caspian and Aral Seas, C. pengoi likely spread to the Great Lakes via ballast water from the Baltic Sea (MacIsaac et al. 1999; Cristescu et al. 2001). As a generalist predator, it competes with native zooplankton and meroplankton such as Leptodora kindtii Focke, 1844, and larval fish, leading to significant ecological and economic impacts (Ojaveer and Lumberg 1995; MacIsaac et al. 1999; Jacobs and MacIsaac 2007; Naumenko and Telesh 2019).
The basic premise of SDMs, namely niche conservatism, is key to understanding the invasion dynamics of C. pengoi. This species has successfully established populations in non-native areas, despite its low genetic diversity. Interestingly, it exhibits high phenotypic variation through cyclomorphosis—environmentally induced changes in cladoceran morphology (Gorokhova et al. 2000; Makarewicz et al. 2001; Litvinchuk and Telesh 2006). This suggests that physiological and morphological adaptability, a trait often seen in cladocerans (Gustafsson et al. 2005), might play a more crucial role in its invasion success than previously thought. Additionally, the limited geographic spread of C. pengoi over decades is noteworthy, as it suggests the potential to thrive in new environments. However, gathering reliable presence/absence data for C. pengoi, is challenging and nearly impossible to maintain globally. Thus, examining the current niches of C. pengoi in the Baltic Sea and North America, compared to its native Caspian region, offers an opportunity to uncover the patterns and biological processes shaping the niches of well-established invasive non-native species. Specifically, it allows us to investigate whether factors other than climatic shifts play a more significant role in determining their establishment and persistence, even at lower abundances (Spear et al., 2021).
Our first objective is to test whether the niche of the species shifts or remains unchanged after it establishes itself in non-native regions (Warren et al. 2008). We propose that niche conservation occurred from native to regional introductions in the Baltic Sea, while niche expansion plays a significant role in the successful establishment of populations overseas. The invasion success of C. pengoi was found to be more influenced by environmental filters rather than by biotic interactions (Golubkov et al. 2020), possibly due to the limited genetic variation between these areas (MacIsaac et al. 1999). By investigating the relative importance of niche conservatism versus niche shift, we aim to determine whether C. pengoi has spread to all potentially suitable habitats in the invaded ranges and, therefore, identify potential areas at risk for further spread. Specifically, we aim to assess whether the invasive ranges predict more similar potential areas compared to currently occupied ranges. The implications of our findings for the use of SDMs as tools for predicting new occurrences of non-native species are also briefly discussed.
Methods
Occurrence and environmental data
We gathered occurrence records of C. pengoi from (i) the Global Biodiversity Information Facility (GBIF 2021); (ii) the US Geological Survey’s Nonindigenous Aquatic Species Database (USGS-NAS; Fuller and Nielson 2015; Benson et al. 2023); and (iii) the Ocean Biodiversity Information System (OBIS; https://obis.org/taxon/234025); and (iv) literature-extracted data, which we searched by title, abstract, and keywords in Scopus and the Web of Science using the search strings: Cercopagis AND (“invas*” OR “non-native” OR “non-indigenous” OR “alien*” OR “introduc*”).
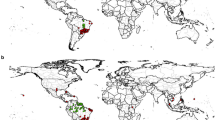
In total, we gathered 1,652 records of C. pengoi in the Ponto-Aralo-Caspian basin and two invasive ranges: ‘Invaded region 1’ (Inv1) in the Baltic Sea and ‘Invaded region 2’ (Inv2) in the Laurentian Great Lakes and Finger Lakes in the eastern North American region. We thinned data with a 10-km buffer around each coordinate using the spThin R library (Aiello-Lammens et al. 2015) to minimise unevenness in sampling efforts, geographical sampling biases, and spatial autocorrelation. As a result, we retained a subset of geographically unique occurrences (Native region = 11, Inv1 = 100, and Inv2 = 63) (Table S1 in Supplementary Information S1).
All bioclimatic variables for the current climatic conditions available from the WorldClim database (http://www.worldclim.org; Hijmans et al. 2005) were gathered at a 2.5 arc-min resolution for the analyses described in sections ‘Niche dynamics’ and ‘Projecting potential distribution in current scenarios’. We pre-selected variables to ensure the use of relevant proxies that better describe C. pengoi distribution and climatic niche as advised as one of the best practices for constructing species distribution and ecological niche models (Araújo et al. 2019). The selection of each variable was based on environmental characteristics that are key drivers of the population dynamics and reproductive patterns of C. pengoi. These factors may account for its successful invasion and its fitness within its native range, as discussed by Golubkov et al. (2020) and Litvinchuk (2021). The relevance of these variables is explained in detail in Table 1.
Niche dynamics
We estimated the amount of niche overlap, i.e. Schoener’s D (Schoener 1970; Warren et al. 2008), based on species abundance and the environmental conditions available for each population (Native region, Inv1, and Inv2), to test niche conservatism hypothesis. If the niche remains consistent throughout the invasion process, spatial models developed using data from the native range of C. pengoi should also indicate suitable conditions in the areas where the species has been introduced. We evaluated niche overlap with multivariate niche similarity tests, i.e. whether one niche is more similar to the others than would be expected by chance, given the available environmental background. Niche conservatism was inferred when the niche similarity test yielded significant values, suggesting that the niches are more similar than what random chance would predict. This means determining whether the climatic niche of one population can more accurately predict another population compared to niches randomly generated from a background region. To thoroughly interpret niche dynamics, we used the density of occurrences in environmental space. This approach helps estimating niche stability (i.e. the proportion of native niche conditions present in the non-native range), assesses niche expansion for insights into species adaptation (i.e. new environmental conditions encountered in the non-native range), and identifies environmental niche unfilling (i.e. the proportion of the native niche not occupied in the non-native range, as detailed by Guisan et al. 2014).
First, we generated an environmental space based on the principal components analysis (PCA) values calculated for the background and occurrence records. We then modelled the species density in the environmental grid, considering the observed occurrence density and the availability of the conditions in the background. We calculated the niche overlap and the partition of the non-overlapped niche among niche unfilling (i.e. niche space that is occupied in the native but unoccupied in the non-native range), expansion (i.e. niche space that is unoccupied in the native but occupied in the invaded range), and stability (i.e. the proportion of the non-native niche overlapping the native niche) (Warren et al. 2008; Broennimann et al. 2012; Guisan et al. 2014).
We extracted values for the four pre-selected bioclimatic variables (bio03, bio10, bio14, and bio15) at a resolution of 2.5 arc-min using the defined backgrounds (Fig. 1A). We randomised the occurrence records for both backgrounds. We generated a buffer of ~ 100 km around the occurrence records to further determine the background available conditions to further apply a PCA for all combined background environmental conditions and generate an environmental space (PCA-env; Broennimann et al. 2012). We calculated the occurrence density within each cell of the environmental space grid for the entire distribution range of the species. Finally, we estimated the environmental space using a smooth kernel density function that considers the geographical conditions available for the studied ranges (Broennimann et al. 2012). A total of 10,000 pseudo-absences were generated, and Schoener’s D was calculated 100 times to produce a null distribution of overlap scores (α = 0.05), which were then compared to the observed value (Warren et al. 2008).
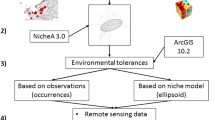
Workflow for (A) species and (B) environmental data preparation, followed by the modelling procedures, is detailed. For niche dynamic analysis (C), a background derived from a Minimum Convex Polygon (MCP), constructed using occurrence records (Native region = 11, Inv1 = 100, and Inv2 = 63), was employed. In reciprocal distribution modelling (B), calibration was performed on one range with subsequent projection into another, and vice versa. Although the native range includes the Ponto-Aralo-Caspian basin and the Black Sea (denoted in blue as ‘Native’), it was not individually utilised in the training dataset for the native range in reciprocal modelling due to data insufficiency. Instead, a comprehensive dataset incorporating all records was employed. The invaded ranges are identified as the Baltic Sea and the Volga rivers in Russia (denoted in orange as ‘Invasive1’) and Northeastern North America in the Great Lakes (denoted in green as ‘Invasive2’)
Projecting potential distribution in current scenarios
We used the same uncorrelated environmental variables used in the niche dynamics analysis in the reciprocal distribution modelling (Table 1). This approach allows identifying which geographic areas share similar climatic conditions between the calibrated and projected ranges. Results from the potential distribution of C. pengoi in both invaded ranges and the native range will uncover the areas not currently occupied (e.g. due to local extinctions or yet not reached) but with suitable climatic conditions for C. pengoi to prosper. This approach would also be suitable to infer the magnitude and direction of possible future range expansions. Our approach followed the workflow (Fig. 1A, C) where the models were calibrated in the native range and projected onto the two non-native ranges (Native region → Inv1, Native region → Inv2). Similarly, we calibrated models in the non-native ranges and projected them onto the native range and from one non-native range to the other (Inv1 → Native region, Inv1 → Inv2, and Inv2 → Inv1, Inv2 → Native region).
The modelling procedure was conducted using the sdm R package (Naimi and Araújo 2016). We used the machine-learning Maximum Entropy algorithm (Maxent; Phillips et al. 2006), one of the most utilised algorithms (Bradie and Leung 2017). This algorithm has good performance and accuracy, being flexible with absence data (Elith et al. 2011), a relevant feature when dealing with invasive species that are plausibly spreading below detection rates (Araújo and Peterson 2012). We generated randomly distributed background points in each calibration area (see Fig. 1) in a proportion of 1:10 (1 presence record to10 background points).
Following evidence-based recommendations, we fine-tuned process to determine the best Maxent settings for each calibration—projected area (All occurrences → Inv1 and Inv2, Inv1 → Inv2, and Inv2 → Inv1). For this procedure, we utilised the framework implemented in the ENMeval R package (Kass et al. 2021), where several configurations of features (L, LQ, LQH, and LQHP) and regularisation multipliers (1, 2, 3, 4, and 5) were tested to provide the best configuration for each model. We retained model settings that presented the lowest ∆AIC (Akaike Information Criterion; Akaike 1973). Models configurations are presented in Table S2 (Supplementary Information S2). We generated 20 replicates evaluated using bootstrapping or subsampling methods selecting 30% of random records for model evaluation. Model performance was estimated using (1) the area under the receiver operating curve (AUC; Fielding and Bell 1997), (2) true skills statistics (TSS; Allouche et al. 2006), and (3) the continuous Boyce index (CBI; Boyce et al. 2002; Hirzel et al. 2006). Lastly, we used the weighted average based on TSS to obtain the consensus model of the probability of occurrence. We considered the threshold that maximises sensitivity and specificity (max sp + se) as the cut-off value (Liu et al. 2013). All analyses were carried out in R language and environment version 4.3.0 (R Core Team 2023) with the ecospat R package (Di Cola et al. 2017).
Results
Niche comparison tests
The environmental niche spaces occupied by C. pengoi in its native and invaded ranges (Inv1 and Inv2) are represented in Fig. 2A–C. The first two PCA axes combined explained 77.1% of the environmental variation (52.1% for the first and 25.7% for the second axis; Fig. 2D). Isothermality (bio3) and precipitation seasonality (bio15) were the most important variables associated with the first axis (Fig. 2E) whereas the second axis was associated with the mean temperature of the warmest quarter (bio10) and precipitation of the driest month (bio14) (Fig. 2F). According to the PCA-env approach, native populations have more similar environmental conditions with Inv1 than with Inv2, which represents a subgroup of the native space (Fig. 3). In contrast, a low proportion of the environmental space in Inv2 is contained in the native space. The environmental space in North America (Inv2) has more adjacent space with the European range (Inv1), also shown by the environmental niche overlap between these ranges (Schoener’s D values; Table 1). The proportion of niche overlap exhibited between all three ranges is shown in Fig. 3, where the dashed lines represent the total background available in each region.
The first two axes of the principal component analysis (PCA), pertaining to the current distribution of Cercopagis pengoi in (A)–(B) invasive ranges and (C) native range, are presented. The PCA outputs for the preselected bioclimatic variables are shown in (D). The importance of variables in each PCA axis is detailed in (E) for the first axis and (F) for the second axis. The continuous line denotes 100% of the available environmental background, while the dashed line signifies the 50% most common conditions
Overlaps in the realised climatic niches of Cercopagis pengoi across all considered ranges are depicted, with niches superimposed upon the available climatic background. The native range is represented in blue (Native); The non-native region Baltic Sea is represented in dark orange (Inv1) and the North American non-native region in green (Inv2). Continuous contours indicate the top 20% of density values, while dashed thin lines outline 100% of the available background in each respective region
The similarity hypothesis test between Native and Inv1 (Table 2; p < 0.05) indicated that these niches were more similar to each other than to 100 randomly selected niches from the Native and Inv1 ranges (Broennimann et al. 2012). The observed overlap, based on Schoener’s D, is significantly higher than what would be expected to occur by chance. However, the similarity test results for Native-Inv2 and Inv1-Inv2 showed a non-significant similarity,suggesting that these niches are less similar than what would be expected by random chance, as detailed in Table 2.
Native and Inv2 had the lowest stability indicating dissimilar environmental conditions between both Ponto-Caspian and North American ranges (1 → 2, 28% and 2 → 1, 15%, Table 2). Furthermore, the higher degree of unfilling between Native region → Inv2 suggests that large portions of unoccupied environments remain available for C. pengoi in North America (unfilling = 85%). The niche dynamics analysis revealed a substantial expansion between the native range and the North American range (Native region → Inv2) at 72%, and between the Baltic and North American invasive ranges (Inv1 → Inv2) at 39%. In contrast, a smaller expansion of 16% was observed between the native range and the Baltic range (Native region → Inv1), as detailed in Table 2.
Reciprocal projections of SDM
Response curves showed that the distribution of C. pengoi is related to regions with less daily temperature variation compared to the annual range, with an optimal isothermality (bio3) slightly below 17%. Cercopagis pengoi displayed different suitability to average temperatures in its invaded ranges (around 16 °C in Inv1 and 20 °C in Inv2), however with similar temperature amplitudes (Fig. 4). Overall, C. pengoi demonstrates higher suitability in relatively stable and moderate hydrological regime, with populations in Inv2 having a slightly higher tolerance for precipitation seasonality compared to the Inv1.
The reciprocal SDMs indicated an overall good performance considering all metrics utilised. Final models trained in all ranges (Native region + Inv1 + Inv2) had AUC 0.92 ± 0.01 (mean ± standard deviation), TSS 0.75 ± 0.03, and CBI 0.91 ± 0.05. Those trained in the Inv1 had AUC 0.92 ± 0.02, TSS 0.75 ± 0.06, and CBI 0.86 ± 0.06. Finally, when trained in Inv2, the model had AUC 0.97 ± 0.01, TSS 0.91 ± 0.04, and Boyce index (CBI) 0.66 ± 0.21. These results of the reciprocal modelling suggest that predictions calibrated in all ranges had an overall better performance than those calibrated only in the invaded regions (Fig. 5A).
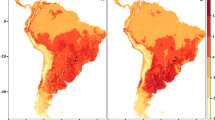
Ensemble predictions for Cercopagis pengoi under the current scenario were derived using a weighted mean of models generated with MaxEnt. The binary suitability maps display the species’ predicted presence based on the maximum sensitivity plus specificity threshold (refer to the ‘Methods’ section for additional details). Colours indicate suitable areas, with red, yellow, and green representing varying degrees of suitability, while unsuitable areas are shown in grey. Maps correspond to predictions based on training data incorporating all occurrences (A), Inv1 (B), and Inv2 occurrences (C). Black dots mark the current distribution of C. pengoi
The potential distribution of C. pengoi, as predicted by models calibrated in Inv1 (Fig. 5B), did not encompass areas currently found in the native range or within Inv2. On the other hand, models calibrated in Inv2 showed higher accuracy in predicting the occurrence of C. pengoi in its native range. These models also indicated more limited and geographically scattered regions in North America than those calibrated with all occurrences (Fig. 5C). The models trained in the invaded ranges displayed similar projected areas in Eurasia. They identified much larger geographic areas as climatically suitable for C. pengoi than its known current distribution, particularly in the Middle East and Russian water bodies. However, the risk of invasion is low in North Africa and Hudson Bay in Canada, as well as in most of the European territory and the Mediterranean Sea.
Discussion
In recent decades, a range of brackish and freshwater habitats in Europe and North America, including the Baltic Sea and the Laurentian Great Lakes, have experienced numerous invasions by species native to the Azov, Black and Caspian Seas. This phenomenon is known as the Ponto-Caspian invasion (Soto et al., 2023). Despite the environmental conditions and shipping frequencies in these regions, these invasions are far more prevalent than expected (Casties and Briski 2019). Euryhalinity, the ability to tolerate a wide range of salinities, is often investigated as a crucial invasiveness trait (Pauli and Briski 2018; Dobrzycka-Krahel et al. 2023). Nevertheless, climate conditions also play a significant role, often triggering sudden surges in invasive populations, specifically by accelerating the hatching of resting eggs (Spear et al. 2021). Our SDM specifically examined Cercopagis pengoi, a notorious high-ranking predator recognised by the IUCN as an impactful invasive species for over two decades (Luque et al. 2014). We sought to quantify the niche dynamics of C. pengoi, thereby enriching knowledge of niche instability as a mechanism behind plankton invasions. Importantly, our models identified regions potentially susceptible to the establishment of C. pengoi, emphasising the urgency of targeted preventive measures.
Niche dynamics
The debate on invasive population dynamics centres around two opposing ideas: one views invasive species as exhibiting niche conservatism, colonising environments akin to their native range, while the other suggests a niche shift occurs during the invasion process (Liu et al. 2020; 2022). In our study, we found support for partially rejecting the niche conservatism hypothesis during the invasion process of C. pengoi. However, invasive populations of C. pengoi demonstrated tendencies towards unfilling or evolution of their ecological niches, native environmental space was more similar to the non-native region of the Baltic Sea (Inv1) than would be expected by chance, a result consistent with niche conservatism. This suggests that the first non-native populations in the Baltic Sea likely originated from the native source range in the Ponto-Caspian region, also indicating a colonisation of populations affected by bottlenecks. This route is also supported by mitochondrial genetic analysis (MacIsaac et al. 1999) and by the low intraspecific genetic diversity commonly found in non-native populations (Gorokhova et al. 2000; Makarewicz et al. 2001; Litvinchuk and Telesh 2006). However, in this plausible initial invasion step, niche comparison analyses indicated that C. pengoi has only partially occupied its ancestral niche, suggesting that future invasions are anticipated.
Significant niche similarity was not observed when comparing the native space with the space of Inv2, nor between Inv1 and Inv2. Rather, a high degree of unfilling (i.e. conditions present in the native range but absent in the invasive range) and expansion (i.e. conditions unique to the invaded range) was evident between the native and North American populations (Inv2), as well as, to a lesser extent, between Inv1 and Inv2. This indicates greater stability, or the ability of the species to maintain its ecological niche over time (Warren et al. 2008; Broennimann et al. 2012). This implies that while the invasion process of Inv1 does not show a significant change in the environmental niche, the subsequent expansion into Inv2 could represent either the still-unfilled conditions in North America (indicating climatic non-equilibrium) or evolutionary processes occurring post-invasion in this region (Broennimann et al. 2012). The greater degree of unfilling observed between the Native region and Inv2, as compared to that between Inv1 and Inv2, might be attributed to dispersal filters rather than biotic resistance. This is because Inv1 is presumably a more intense source of propagules to Inv2, potentially sustaining ongoing propagule pressure. In other words, there is likely higher anthropogenic dispersal facilitated by the more active commercial route between the Baltic Sea and North America (Hudgins et al. 2023). Given that cladocerans rely on wind, drift, and other animals for the dispersal of their propagules (adult form or resting stages) (Moreno et al. 2019), it is predominantly human-assisted dispersal that facilitates their widespread distribution (Incagnone et al. 2015; Kotov et al. 2022).
In the study by Torres et al. (2018), a significant shift in the ecological niches of most freshwater invertebrates, including C. pengoi, was observed during their transition from native regions to potential new invasive spots in New Zealand. This shift in niche dynamics, as evidenced by the metrics in Table 2, could be attributed to rapid evolutionary changes or adaptations to novel environmental conditions. Such adaptations are particular features of cladocerans, which have short life cycles and well-known adaptive traits (LaMontagne and McCauley 2001; Gustafsson et al. 2005). However, the role of phenotypic plasticity in the establishment of C. pengoi also warrants consideration. Phenotypic plasticity, the ability of an organism to alter its phenotype in response to environmental variations, might be a key factor in the establishment of the species, as suggested in the invasion process of North American lakes by C. pengoi (MacIsaac et al. 1999). Indeed, if plasticity is a significant driver, the invasion pathway characterised by the sequence Native region > Inv1 > Inv2 might not strictly adhere to a pattern of pre-adaptation.
Northern regional and intercontinental range expansion
Suitable but not yet occupied environments can become time-bombs for sudden outbreaks of C. pengoi, as already shown for ecologically similar invasive cladocerans (e.g. Bythotrephes longimanus; Spear et al. 2021). Additionally, environmental non-equilibrium, as demonstrated here for C. pengoi, can undermine the accuracy of invasion risk assessments and thus deserves special attention from monitoring programs (Cutter et al., 2023; Maxson et al., 2023). These assessments can identify whether management biases or delays—attributed to the premise of the absence of invasive species due to non-detection—are plausible, given their ability to thrive in novel environments (Capinha et al. 2011; Gallardo et al. 2013; Torres et al. 2018; Macêdo et al. 2021). Curiously, C. pengoi populations are not established elsewhere in the world outside of the areas investigated in this study. In this sense, predicting the shifts in species ranges in response to environmental change is critical for developing timely conservation and mitigation strategies (i.e. anticipation or biocontrol; Dinis et al. 2020), and targeting costly invasive species (Cuthbert et al. 2021; Macêdo et al. 2022b), as inaction has been proven to be deleterious to global economies (Ahmed et al. 2022).
Our projections indicate improved model performances when based on both native and invasive ranges (Loo et al. 2007; Jiménez-Valverde et al. 2011; Macêdo et al. 2021). Invasive populations of C. pengoi may be adapted to different average temperatures while maintaining a consistent preference for limited daily temperature variability. Also, the responses to precipitation suggest that invasive populations have different drought resistance. These results suggest a degree of ecological plasticity in adapting to the mean thermal conditions of an area while maintaining a selective affinity for a narrow range of temperature fluctuations, which likely contributes to its invasive success in distinct yet climatically divergent regions.
Surpassing all occupancy restrictions, surveillance priority areas can be pointed out, aiming for more efficient control strategies for C. pengoi invasion. Starting from areas close to the source populations in North America and extending northward, e.g. the plains of Ontario and Quebec, Canada. Also, the predicted expansion southward into Illinois, possibly reaching even more southernmost locations in North America through natural downstream movements. For instance, Maxson et al. (2023) recently reported new records of C. pengoi in the Mississippi River Basin, which represents so far the southernmost record in the North American range. In Europe, we suggest higher efforts in Northern countries (Sweden, Finland, Denmark, and Norway), but primarily in Russian water bodies that have been invaded by zooplankton organisms through global trade (DiBacco et al. 2012; Casas-Monroy et al. 2015), including recent geographic range expansion of C. pengoi into Russian waterways (Lazareva 2019a, 2019b). The suitable areas for C. pengoi invasions indicated by our models are also targets for novel commercial ship routes in the Arctic (McCarthy et al. 2019), indicating areas where this cladoceran is currently absent (e.g. the Mediterranean Sea; Zenetos and Galanidi 2020). However, false negatives are possible as biodiversity in these regions has not been completely assessed, as new species are continuously being described (e.g. Alonso et al. 2021). Since methods of sterilisation of ballast water tanks can fail to reduce propagule pressure and instead trigger resting eggs hatching (Lin et al. 2020), implementing constant monitoring programs should be considered to mitigate both ecological and economic impacts, mainly to fish and fisheries (Macêdo et al. 2022b).
Conclusion
Species distribution modelling can provide insights into the mechanisms driving the invasion establishment of different populations of C. pengoi, beyond expectations of widespread niche conservatism of non-native species. Although relatively geographically restricted in invaded ranges, C. pengoi is not climatically constrained to the localities of its known current distribution, suggesting that more significant portions could be occupied if control policies weaken, or under novel environmental conditions that may trigger a population outbreak. Also, niches are neither similar nor stable between invasive ranges in Europe and North America, and introductions from these sources are possible or already ongoing but not tracked due to detection limitations. We suggest monitoring efforts should conduct samplings in bottom sediments due to resting egg bank formation for rapid detection and action, as well as developing protocols for ballast water sterilisation. In doing so, the invasion process of C. pengoi could be elucidated at local and regional scales, avoiding impacts on biodiversity and economic activities.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmed DA, Hudgins EJ, Cuthbert RN, Kourantidou M, Diagne C et al (2022) Managing biological invasions: the cost of inaction. Biol Invasions 24:1927–1946. https://doi.org/10.1007/s10530-022-02755-0
Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP et al (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545. https://doi.org/10.1111/ecog.01132
Akaike, H (1973) Information theory and an extension of the maximum likelihood principle. - In: Petrov BN, Caski F (eds) Proceedings of the second international symposium on information theory, Budapest, Hungary, pp 267–281
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Alonso M, Neretina AN, Ventura M (2021) Ceriodaphnia smirnovi (Crustacea: Cladocera), a new species from the Mediterranean Region, and a phylogenetic analysis of the commonest species. Zootaxa 4974. https://doi.org/10.11646/zootaxa.4974.1.1
Araújo MB, Anderson RP, Barbosa AM, Beale CM, Dormann CF et al (2019) Standards for distribution models in biodiversity assessments. Sci Adv 5. https://doi.org/10.1126/sciadv.aat4858
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12:20150623. https://doi.org/10.1098/rsbl.2015.0623
Benson A, Maynard E, Raikow D, Larson J, Makled TH, Fusaro A (2023) Cercopagis pengoi: U.S. geological survey, nonindigenous aquatic species database, Gainesville, FL. https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=163. Accessed 30 May 2023
Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA (2002) Evaluating resource selection functions. Ecol Modell 157:281–300. https://doi.org/10.1016/S0304-3800(02)00200-4
Bradie J, Leung B (2017) A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J Biogeogr 44:1344–1361. https://doi.org/10.1111/jbi.12894
Briski E, Bailey SA, Casas-Monroy O, DiBacco C, Kaczmarska I et al (2012) Relationship between propagule pressure and colonization pressure in invasion ecology: a test with ships’ ballast. Proc R Soc B: Biol Sci 279:2990–2997. https://doi.org/10.1098/rspb.2011.2671
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L et al (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21:481–497. https://doi.org/10.1111/j.1466-8238.2011.00698.x
Capinha C, Leung B, Anastácio P (2011) Predicting worldwide invasiveness for four major problematic decapods: an evaluation of using different calibration sets. Ecography 34:448–459. https://doi.org/10.1111/j.1600-0587.2010.06369.x
Card DC, Perry BW, Adams RH, Schield DR, Young AS et al (2018) Novel ecological and climatic conditions drive rapid adaptation in invasive Florida Burmese pythons. Mol Ecol 27:4744–4757. https://doi.org/10.1111/mec.14885
Casas-Monroy O, Linley RD, Adams JK, Chan FT, Drake DAR et al (2015) Relative invasion risk for plankton across marine and freshwater systems: examining efficacy of proposed international ballast water discharge standards. PLoS ONE 10:e0118267. https://doi.org/10.1371/journal.pone.0118267
Casties I, Briski E (2019) Life history traits of aquatic non-indigenous species: freshwater vs. marine habitats. Aquat Invasions 14:566–581. https://doi.org/10.3391/ai.2019.14.4.01
Cuthbert RN, Pattison Z, Taylor NG et al (2021) Global economic costs of aquatic invasive alien species. Sci Total Environ 775:145238. https://doi.org/10.1016/j.scitotenv.2021.145238
Cutter ZA, Mihuc TB, Myers LW (2023) Invasion of Bythotrephes longimanus and Cercopagis pengoi in Lake Champlain: impacts on the native zooplankton community. Diversity 15(11):1112. https://doi.org/10.3390/d15111112
Cristescu ME, Hebert PD, Witt JD, MacIsaac HJ, Grigorovich IA (2001) An invasion history forCercopagis pengoi based on mitochondrial gene sequences. Limnol Oceanogr 46(2):224–229
Di Cola V, Broennimann O, Petitpierre B, Breiner FT, D’Amen M et al (2017) ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography 40:774–787. https://doi.org/10.1111/ecog.02671
DiBacco C, Humphrey DB, Nasmith LE, Levings CD (2012) Ballast water transport of non-indigenous zooplankton to Canadian ports. ICES J Mar Sci 69:483–491. https://doi.org/10.1093/icesjms/fsr133
Dinis M, Vicente JR, de Sá NC, López-Núñez FA, Marchante E et al (2020) Can niche dynamics and distribution modeling predict the success of invasive species management using biocontrol? Insights from Acacia longifolia in Portugal. Front Ecol Evol 8. https://doi.org/10.3389/fevo.2020.576667
Dobrzycka-Krahel A, Stepien CA, Nuc Z (2023) Neocosmopolitan distributions of invertebrate aquatic invasive species due to euryhaline geographic history and human-mediated dispersal: Ponto-Caspian versus other geographic origins. Ecol Process 12:2. https://doi.org/10.1186/s13717-022-00412-x
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE et al (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49. https://doi.org/10.1017/S0376892997000088
Gallardo B, zu Ermgassen PSE, Aldridge DC (2013) Invasion ratcheting in the zebra mussel (Dreissena polymorpha) and the ability of native and invaded ranges to predict its global distribution. J Biogeogr 40:2274–2284. https://doi.org/10.1111/jbi.1217
GBIF (2021) GBIF Occurrence Download. https://doi.org/10.15468/dl.us7urc
Golubkov MS, Litvinchuk LF, Golubkov SM (2020) Effects of environmental gradients on the biomass of alien Cercopagis pengoi in the Neva Estuary. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.573289
Gorokhova E, Aladin N, Dumont HJ (2000) Further expansion of the genus Cercopagis (Crustacea, Branchiopoda, Onychopoda) in the Baltic Sea, with notes on the taxa present and their ecology. Hydrobiologia 429:207–218. https://doi.org/10.1023/A:1004004504571
Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C et al (2014) Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29:260–269. https://doi.org/10.1016/j.tree.2014.02.009
Gustafsson S, Rengefors K, Hansson L-A (2005) Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 86:2561–2567. https://doi.org/10.1890/04-1710
Han L, Zhang Z, Tu W, Yang Z, Shuai Y et al (2023) Preferred prey reduce species realized niche shift and improve range expansion prediction. Sci Total Environ 859:160370. https://doi.org/10.1016/j.scitotenv.2022.160370
Haubrock PJ, Cuthbert RN, Hudgins EJ, Chang CH, Iannella M et al (2022) Geographic and taxonomic trends of rising biological invasion costs. Sci Total Environ 817:152948. https://doi.org/10.1016/j.scitotenv.2022.152948
Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB (2015) Aquatic invasive species: challenges for the future. Hydrobiologia 750:147–170. https://doi.org/10.1007/s10750-014-2166-0
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high-resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. https://doi.org/10.1002/joc.1276
Hirzel AH, Le Lay G, Helfer V, Randin C, Guisan A (2006) Evaluating the ability of habitat suitability models to predict species presences. Ecol Modell 199:142–152. https://doi.org/10.1016/j.ecolmodel.2006.05.017
Hudgins EJ, Cuthbert RN, Haubrock PJ, Turbelin AJ, Chang CH et al (2023) Unevenly distributed biological invasion costs among origin and recipient regions. Nat Sustain 6:1113–1124. https://doi.org/10.1038/s41893-023-01124-6
Hui C, Pyšek P, Richardson DM (2023) Disentangling the relationships among abundance, invasiveness and invasibility in trait space. npj Biodiversity 2:13. https://doi.org/10.1038/s44185-023-00019-1
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18. https://doi.org/10.1111/j.1365-2664.2008.01600.x
Incagnone G, Marrone F, Barone R et al (2015) How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750:103–123. https://doi.org/10.1007/s10750-014-2110-3
Jacobs MJ, MacIsaac HJ (2007) Fouling of fishing line by the waterflea Cercopagis pengoi: a mechanism of human-mediated dispersal of zooplankton? Hydrobiologia 583:119–126. https://doi.org/10.1007/s10750-006-0487-3
Jiménez-Valverde A, Peterson AT, Soberón J, Overton JM, Aragón P et al (2011) Use of niche models in invasive species risk assessments. Biol Invasions 13:2785–2797. https://doi.org/10.1007/s10530-011-9963-4
Kass JM, Muscarella R, Galante PJ, Bohl CL, Pinilla-Buitrago GE et al (2021) ENMeval 2.0: redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol Evol 12:1602–1608. https://doi.org/10.1111/2041-210X.13628
Kotov AA, Karabanov DP, Van Damme K (2022) Non-iIndigenous Cladocera (Crustacea: Branchiopoda): fFrom a fFew nNotorious cCases to a pPotential gGlobal fFaunal mMixing in aAquatic eEcosystems. Water (Basel) 14:2806. https://doi.org/10.3390/w14182806
LaMontagne JM, McCauley E (2001) Maternal effects in Daphnia: what mothers are telling their offspring and do they listen? Ecol Lett 4:64–71. https://doi.org/10.1046/j.1461-0248.2001.00197.x
Lazareva VI (2019a) Spreading of aAlien zZooplankton sSpecies of Ponto-Caspian oOrigin in the rReservoirs of the Volga and Kama Rivers. Russ J Biol Invasions 10:328–348. https://doi.org/10.1134/S2075111719040040
Lazareva VI (2019b) New rRecords of the Ponto-Caspian pPredatory cladoceran Cercopagis pengoi (Ostroumov, 1891) (Crustacea, Onychopoda) in the wWater rReservoirs of the Kama and Volga Rivers. Inland Water Biol 12:290–297. https://doi.org/10.1134/S1995082919030118
Lin Y, Zhan A, Hernandez MR, Paolucci E, MacIsaac HJ et al (2020) Can chlorination of ballast water reduce biological invasions? J Appl Ecol 57:331–343. https://doi.org/10.1111/1365-2664.13528
Litvinchuk LF, Telesh IV (2006) Distribution, population structure, and ecosystem effects of the invader Cercopagis pengoi (Polyphemoidea, Cladocera) in the Gulf of Finland and the open Baltic Sea. Oceanologia 48:243–257
Litvinchuk LF (2021) Long-term and seasonal dynamics of the population of Cercopagis pengoi (Ostroumov 1891) (Cladocera, Onychopoda) in the eastern part of the Gulf of Finland, Baltic Sea. Biol Bull 48:1202–1209. https://doi.org/10.1134/S1062359021080197
Liu C, White M, Newell G (2013) Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr 40:778–789. https://doi.org/10.1111/jbi.12058
Liu C, Wolter C, Courchamp F, Roura-Pascual N, Jeschke JM (2022) Biological invasions reveal how niche change affects the transferability of species distribution models. Ecology 103. https://doi.org/10.1002/ecy.3719
Liu C, Wolter C, Xian W, Jeschke JM (2020) Most invasive species largely conserve their climatic niche. Proc Natl Acad Sci 117:23643–23651. https://doi.org/10.1073/pnas.2004289117
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228. https://doi.org/10.1016/j.tree.2005.02.004
Loo SE, Mac Nally R, Lake PS (2007) Forecasting New Zealand mudsnail invasion range: model comparisons using native and invaded ranges. Ecological Applications 17:181–189. https://doi.org/10.1890/1051-0761(2007)017[0181:FNZMIR]2.0.CO;2
Luque GM, Bellard C, Bertelsmeier C, Bonnaud E, Genovesi P et al (2014) The 100th of the world’s worst invasive alien species. Biol Invasions 16:981–985. https://doi.org/10.1007/s10530-013-0561-5
Macêdo RL, Russo P, Corrêa RF, Rocha O, Santos LN et al (2021) The drifting dinoflagellate Ceratium furcoides (Levander) Langhans 1925: fundamental niche shift during global invasion. Hydrobiologia 848:2105–2117. https://doi.org/10.1007/s10750-020-04495-5
Macêdo RL, Sousa FDR, Dumont HJ, Rietzler AC, Rocha O, Elmoor-Loureiro LMA (2022a) Climate change and niche unfilling tend to favor range expansion of Moina macrocopa Straus 1820, a potentially invasive cladoceran in temporary waters. Hydrobiologia 849(17-18):4015–4027. https://doi.org/10.1007/s10750- 022-04835-7
Macêdo RL, Franco ACS, Kozlowsky-Suzuki B, Mammola S, Dalu T, Rocha O (2022b) The global social-economic dimension of biological invasions by plankton: Grossly underestimated costs but a rising concern for water quality benefits? Water Res 222:118918
MacIsaac HJ, Grigorovich IA, Hoyle JA, Yan ND, Panov VE et al (1999) Invasion of Lake Ontario by the Ponto-Caspian predatory cladoceran Cercopagis pengoi. Can J Fish Aquat Sci 56:1–5. https://doi.org/10.1139/cjfas-56-1-1
Makarewicz JC, Grigorovich IA, Mills E, Damaske E, Cristescu ME et al (2001) Distribution, fecundity, and genetics of Cercopagis pengoi (Ostroumov) (Crustacea, Cladocera) in Lake Ontario. J Great Lakes Res 27:19–32. https://doi.org/10.1016/S0380-1330(01)70620-X
Maxson KA, Whitten AL, Harris BS, Happel A, Butler SE, Parkos III JJ, Casper AF, Lamer JT (2023) First records of the fishhook waterflea Cercopagis pengoi Ostroumov, 1891 in the Mississippi River Basin, Illinois Waterway, USA. BioInvasions Rec 12(4):1079–1087.https://doi.org/10.3391/bir.2023.12.4.20
McCarthy AH, Peck LS, Hughes KA, Aldridge DC (2019) Antarctica: tThe final frontier for marine biological invasions. Glob Chang Biol 25:2221–2241. https://doi.org/10.1111/gcb.14600
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci 98:5446–5451. https://doi.org/10.1073/pnas.091093398
Moreno E, Pérez-Martínez C, Conde-Porcuna JM (2019) Dispersal of rotifers and cladocerans by waterbirds: seasonal changes and hatching success. Hydrobiologia 834:145–162. https://doi.org/10.1007/s10750-019-3919-6
Naimi B, Araújo MB (2016) sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39:368–375. https://doi.org/10.1111/ecog.01881
Naumenko EN, Telesh IV (2019) Impact of the iInvasive sSpecies Cercopagis pengoi (Ostroumov, 1891) on the sStructural and fFunctional oOrganization of zZooplankton in the Vistula Lagoon of the Baltic Sea. Russ J Biol Invasions 10:246–257. https://doi.org/10.1134/S2075111719030081
Ojaveer H, Lumberg A (1995) On the role of Cercopagis (Cercopagis pengoi Ostroumov) in Parnu Bay and the NE part of the Gulf of Riga ecosystem. Proc Estonian Acad Sci Ecol 5:20. https://doi.org/10.3176/ecol.1995.1/2.03
Pauli N-C, Briski E (2018) Euryhalinity of Ponto-Caspian invaders in their native and introduced regions. Aquat Invasions 13:439–447. https://doi.org/10.3391/ai.2018.13.4.02
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190:231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
R Core Team (2023) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. https://www.R-project.org/
Ricciardi A (2007) Are mModern bBiological iInvasions an uUnprecedented fForm of gGlobal cChange? Conserv Biol 21:329–336. https://doi.org/10.1111/j.1523-1739.2006.00615.x
Schoener TW (1970) Nonsynchronous sSpatial oOverlap of lLizards in pPatchy hHabitats. Ecology 51:408–418. https://doi.org/10.2307/1935376
Soberón J, Nakamura M (2009) Niches and distributional areas: cConcepts, methods, and assumptions. Proc Natl Acad Sci 106:19644–19650. https://doi.org/10.1073/pnas.0901637106
Soto I, Cuthbert RN, Ricciardi A, Ahmed DA, Altermatt FF et al (2023) The faunal Ponto-Caspianization of central and western European waterways. Biol Invasions 25:2613–2629. https://doi.org/10.1007/s10530-023-03060-0
Spear MJ, Walsh JR, Ricciardi A, Vander ZMJ (2021) The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. Bioscience 71:357–369. https://doi.org/10.1093/biosci/biaa168
Tingley R, Vallinoto M, Sequeira F, Kearney MR (2014) Realized niche shift during a global biological invasion. Proc Natl Acad Sci 111:10233–10238. https://doi.org/10.1073/pnas.1405766111
Torres U, Godsoe W, Buckley HL, Parry M, Lustig A et al (2018) Using niche conservatism information to prioritize hotspots of invasion by non-native freshwater invertebrates in New Zealand. Divers Distrib 24:1802–1815. https://doi.org/10.1111/ddi.12818
Walsh JR, Carpenter SR, Vander Zanden MJ (2016) Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc Natl Acad Sci 113:4081–4085. https://doi.org/10.1073/pnas.1600366113
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Wiens JJ, Ackerly DD, Allen AP et al (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324. https://doi.org/10.1111/j.1461-0248.2010.01515.x
Zenetos A, Galanidi M (2020) Mediterranean non indigenous species at the start of the 2020s: recent changes. Mar Biodivers Rec 13:10. https://doi.org/10.1186/s41200-020-00191-4
Acknowledgements
RLM thanks PPGERN (Graduate Program in Ecology and Natural Resources, UFSCAR, Brazil). ACSF thanks the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (postdoctoral fellowship to ACSF, E-26/202.423/2019). DPS is supported by a productivity grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant number: (Process N.º: 307514/2023-4) and research resources from Fundação de Apoio à Pesquisa do Estado de Goiás (FAPEG; Process N.º: 202310267001380).
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by Coordination of Improvement of Higher Education Personnel (Capes)—Finance Code 001. RLM was supported by the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Contributions
RLM conceived the research idea; RLM and GK surveyed and compiled the data; RLM, BV, GK, and DS performed analyses of SDM; RLM wrote the first draft; RLM, DS, GK, ACSF, PJH, SM, and OR revised the first draft and added parts in the final version of the manuscript. The authors have extensively discussed the manuscript, addressing valuable comments from their different field perspectives and revising the final document.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Dror Angel
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Is Cercopagis pengoi, deemed one of the world’s worst invasive species, being effectively controlled or is its expansion going unnoticed?
• Through the integration of multivariate analyses and predictive modelling, we examined its invasion dynamics in Europe and North America.
• Niche unfilling and expansion underscores its potential to invade new environments, either unoccupied or previously unnoticed.
• Predictive models based solely on the species’ known invasive ranges underestimate the full extent of its potential distribution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macêdo, R.L., Klippel, G., Silva, D.P. et al. Intercontinental invasion dynamics of Cercopagis pengoi, an IUCN-listed planktonic invasive species. Reg Environ Change 24, 78 (2024). https://doi.org/10.1007/s10113-024-02244-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-024-02244-1