Abstract
Non-excisional laser therapies are emerging treatment for grades II and III hemorrhoidal disease (HD). However, so far, their efficiency is based on low-level evidence. Therefore, we aimed to systematically review the efficiency of non-excisional laser therapies for HD. MEDLINE/Pubmed, Web of science, Embase, and Cochrane were searched from database implementation until the April 17th, 2020. We included studies reporting at least one of surgical indicators of postoperative outcomes of laser therapies, encompassing laser hemorrhoidoplasty (LH) and hemorrhoidal laser procedure (HeLP). Fourteen studies describing LH and HeLP were included, representing 1570 patients. The main intraoperative complication was bleeding (0–1.9% of pooled patients for LH, 5.5–16.7% of pooled patients for HeLP). Postoperative complications occurred in up to 64% of patients after LH and 23.3% after HeLP. Resolution of symptoms ranged between 70 and 100% after LH and between 83.6 and 90% after HeLP. Moreover, four randomized controlled trials included in our review reported similar resolution after LH compared with hemorrhoidectomy or mucopexy and after HeLP compared with rubber band ligation. Recurrence rate was reported to range between 0 and 11.3% after LH and between 5 and 9.4% after HeLP. When compared with hemorrhoidectomy, LH showed conflicting results with one randomized controlled trial reporting similar recurrence rate, but another reporting decreased recurrences associated with hemorrhoidectomy. Laser therapies showed lower postoperative pain than hemorrhoidectomy or rubber band ligation. LH and HeLP are safe and effective techniques for the treatment of grades II and III HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemorrhoidal disease (HD) is frequent, with an estimated prevalence of 4.4% among the US population [1]. HD is the consequence of an increased inflow into the superior rectal artery, which causes dilatation of the hemorrhoidal plexus. Moreover, degradation of the supportive tissue results in sliding down of hemorrhoids [2]. Hemorrhoids are classified as grade I when they are seen during anoscopy as congested veins, grade II when they prolapse but spontaneously reduce, grade III when they prolapse and need manual reduction, and grade IV when they are irreducible [3]. HD, defined as symptomatic hemorrhoids, can present with pain, itching, bleeding, discharge, or prolapse [4].
Initial treatment of HD consists of lifestyle modifications and administration of phlebotonics. After failure of conservative management, HD is treated with interventional therapies [4]. Open hemorrhoidectomy (HC) was first described in 1937 by Milligan-Morgan [5] and is still considered as the gold standard interventional therapy for advanced stages of HD. However, significant postoperative pain and complications were associated with excision of hemorrhoidal tissue. Therefore, various non-excisional therapies have been developed, such as rubber band ligation (RBL), mucopexy (MP), and more recently laser therapies [4, 6].
Non-excisional laser therapy was initially described in 1998 by Barr et al. [7] with an experimental animal study. Administration of a pulsed laser energy to the submucosal pig rectal tissue allowed coagulation of vessels, with limited damage to the surrounding tissue. Latter, non-excisional laser therapy was applied in humans, with laser hemorrhoidoplasty (LH) first described in 2007 by Karahaliloglu et al. [8]. During LH, a laser fiber is introduced through a skin incision at the hemorrhoidal base, and hemorrhoidal cushions are coagulated. Hemorrhoidal laser procedure (HeLP) constitutes another non-excisional laser therapy for the treatment of HD, first described in 2009 by Salfi et al. [9]. During this procedure, a Doppler identifies the terminal branches of the superior rectal artery, which are coagulated with a pulsed laser energy. Both techniques allow obliteration and retraction of the hemorrhoidal plexus. Moreover, they were shown to be safe and effective for the treatment of HD [8, 9].
Therefore, non-excisional laser therapies constitute interventional therapies for the treatment of HD. However, their recommendation is based on low level of evidence [4, 6]. For the purpose of strengthening the evidence for the benefits of laser therapies, we aimed to systematically review the outcomes of LH and HeLP for the treatment of HD. According to population, intervention, comparison, outcome (PICO) framework, our question was: in patients with HD undergoing non-excisional laser therapies, what are the postoperative outcomes?
Materials and methods
This systematic review adheres to the recommendations of the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement [10] (Supplementary Table 1).
Literature search and study selection
Human studies written in English published before April 17th, 2020 were looked for in MEDLINE/Pubmed, Web of science, Embase, and Cochrane. The search strategy was designed and independently conducted by two authors (GL, EL). The following medical search headings and keywords were used: “hemorrhoids” in MeSH terms; and “haemorrhoid*” OR “hemorrhoid*” OR “hemorrhoidal disease” OR “haemorrhoidal disease” AND “laser” OR “laser hemorrhoidoplasty” OR “hemorrhoidal laser procedure” in non-MeSH terms. The reference lists of included articles were further screened for additional eligible publications.
Outcomes of interest
The aim of the study was to systematically review the outcomes of laser therapies for the treatment of HD, including LH and HeLP.
Primary endpoints were the surgical indicators of postoperative outcomes, including
-
Improvement, defined as postoperative decrease of HD symptoms or grade adapted from the Goligher classification [3];
-
Persistence, defined as postoperative presence of symptoms or prolapse;
-
Resolution, defined as postoperative absence of symptoms or prolapse;
-
Recurrence, defined as reappearance of HD, after a resolution;
-
Reoperation, defined as any procedure performed for HD after the laser therapy.
Secondary endpoints included
-
Perioperative characteristics;
-
Postoperative pain and return to normal activities;
-
Intraoperative and postoperative complications, defined as any deviation from the normal postoperative course. Therefore pain, tenesmus, and dyschezia that resolved spontaneously without treatment were not considered as complications.
Inclusion criteria
Original publications were eligible only if they fulfilled the following criteria: (i) they reported outcomes of laser therapy for HD and (ii) they reported at least one of the primary endpoint. Articles were included regardless of the design and the size of the study population.
Exclusion criteria
The exclusion criteria were as follows: studies reporting (i) laser hemorrhoidectomy or infrared therapy; (ii) laser therapy performed with an associated procedure or for another anorectal pathology than HD; and (iii) conference abstracts, protocols, and editorials.
Data extraction
Two reviewers (GL, EL) extracted the following data: general and methodological information of the study, baseline characteristics of the study population, surgical indicators of postoperative outcomes, perioperative characteristics, complications, postoperative pain, and return to normal actives. Details of extracted data are reported in the Supplementary Table 2.
Results
Literature search and study characteristics
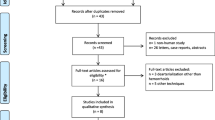
The initial search identified 1031 studies. After title and abstract review, 901 studies were excluded. The remaining 130 studies were fully reviewed. Of these, 117 were excluded because they reported laser hemorrhoidectomy (42 studies), infrared therapy (68 studies), associated procedure (three studies), or laser therapy for other pathology than HD (four studies). Reviewing of references identified one study [9]. Finally, 14 studies [8, 9, 11,12,13,14,15,16,17,18,19,20,21,22] published between 2007 and 2020 were eligible for our review: seven studies [8, 11,12,13,14,15,16] reported LH, and seven studies [9, 17,18,19,20,21,22] reported HeLP (Fig. 1). There were 10 cohorts (seven prospective [11, 12, 17,18,19,20,21], two retrospective [8, 9], one not specified [13]), three randomized controlled trials (RCT) comparing LH with HC [14,15,16] and MP [16], and one RCT comparing HeLP with RBL [22]. Of the 1540 patients included in these studies, majority were classified as suffering from grades II and III HD (35.8% and 33.6%, respectively), while grades I and IV HD patients were less frequent (5.1% and 0.4%, respectively, grade unavailable for 25.1% of cases). The studies’ characteristics and patient demographic details are depicted in Table 1.
Perioperative characteristics
Laser hemorrhoidoplasty
LH was performed under general anesthesia [11, 14, 16], spinal anesthesia [11, 15], local anesthesia with [12, 13] or without [8] sedation, or without anesthesia [8] (Table 2). Antibioprophylaxis was administered in three studies [12, 14, 16]. The operation time was reported in three observational cohorts [8, 11, 12] and three RCTs [14,15,16], which ranged from 5 [11] to 40.4 min [14]. Moreover, the three RCTs [14,15,16] showed a significantly shorter operation time with LH versus open HC (mean 33.1 min ± 7.3 versus mean 52.6 min ± 15.6, p < 0.001 [14]; mean 30.6 min ± 4.9 versus mean 50.5 min ± 12.1, p < 0.001 [15]; mean 15 min ± 5.6 versus mean 29 min ± 10.3, p < 0.001 [16], respectively). Four studies [11,12,13, 15] described the hospitalization duration, which ranged from 3 [13] to 48 h [12] and was similar to open HC [15].
Hemorrhoidal laser procedure
HeLP was performed under topical anesthesia [19, 21], local anesthesia [21], spinal anesthesia [21], sedation [19,20,21], or without anesthesia [9, 17, 18, 20, 22] (Table 3). Four studies prescribed preoperative enema [18, 19, 21, 22] and/or antibioprophylaxis [18,19,20,21]. The operation time ranged from 7 [20, 21] to 40 min [18] and was similar to RBL [22] (HeLP: median 10 min, range 7.8–11.2 versus RBL: median 8 min, range 5.8–9.6, p = 0.96). Hospitalization duration was up to 24 h [17,18,19,20], but was not described in three studies [9, 21, 22].
Postoperative pain and return to normal activities
Postoperative pain was significantly lower after LH compared with HC [13, 15, 16] and resulted in a shorter return to normal activities [15, 16]. Early postoperative visual analog score (VAS) was also decreased with HeLP compared with RBL [22] (mean 1.1 versus 2.9, p < 0.001, respectively). Details of postoperative pain and return to normal activities are depicted in Table 4.
Intraoperative and postoperative complications
Intraoperative complications
Bleeding was the only intraoperative complication reported. It was reported by four studies [8, 12, 14, 15] describing LH and for all studies [9, 17,18,19,20,21,22] describing HeLP (Table 5). Its incidence ranged from 0 [12] to 1.9% [8] and from 5.5 [20] to 16.7% [22], respectively. Two RCTs [14, 15] reported a significant lower blood loss volume during LH versus HC (mean 12.4 ml ± 4.5 versus 22.8 ml ± 8.3, p < 0.001 [14]; mean 15.5 ml ± 4.8 versus mean 36.5 ml ± 7.2, p < 0.001 [15], respectively). However, in the RCT by Giamundo et al. [22], the intraoperative blood loss was similar for HeLP versus RBL (16.7% versus 10%, p = 0.12, respectively).
Postoperative complications
All studies reported postoperative complications (Table 5). The incidence of complications ranged between 0 [16] and 64% [11] after LH and between 0 [17, 18] and 23.3% [22] after HeLP. The most common reported complication was bleeding (range 0–64% after LH and 0–23.3% after HeLP), which resolved with suture [11], packing and/or haemostatic drug [15, 21], conservative therapy [14], or without treatment [12, 20, 22]. Further complications were thrombosis (range 6.7–10% after LH [14, 15], range 1.4–7.8% after HeLP [19, 21]), infection (up to 0.6% after LH [11], not reported after HeLP), and urinary retention (up to 3.3% after LH [14], not reported after HeLP). The latter was significantly increased after HC versus LH (13.3% versus 0%, p = 0.038, respectively) [15]. Other complications reported after LH included mucosal damage (0.9%, treated with ligature) [8], edema (2.3%) [11], burn lesion (26.7%) [13], and skin tag (33.3%) [13]. Other complications reported after HeLP included anismus (1.4%) [21] and sensation of incomplete evacuation (3.1%, which did not require any treatment) [21].
Surgical indicators of postoperative outcomes
Surgical indicators of postoperative outcomes are detailed in Table 6. The postoperative follow-up duration ranged between 1 [13] and 12 months [8, 11, 14, 16] after LH and between 1 [17] and a mean of 35.4 months [19] after HeLP.
HD downgrading
HD downgrading for at least one grade derived from the Goligher classification was reported by four studies after HeLP [17, 18, 20, 22]. It was 80% at 6 months [20, 22], 77% at a mean of 5.8 months [17], and > 85% at 15 months [18]. In the RCT by Giamundo et al. [22], HD grade was significantly decreased after HeLP compared with RBL (80% versus 40% at 6 months, p < 0.001, respectively). No study reported HD downgrading after LH.
Symptom improvement
Improvement of HD symptoms was reported by four studies [9, 17,18,19] after HeLP and ranged from 85% at a median of 15 months [18] to 91.7% at a mean of 5.8 months [17]. No study reported symptom improvement after LH.
Persistence
Symptomatic ± prolapse persistence was 0% at 3 months [15] and 7% of at a mean of 5.8 months [17] after LH. After HeLP, two studies [19, 21] reported 10% of recurrences up to 26 postoperative months.
Resolution
Resolution of hemorrhoidal prolapse was reported in 60.4% of patients at 1 month after LH [13] and 76.9% of patients at a mean of 26.3 months after HeLP [21]. After LH, resolution of symptoms was 100% at 3 months [15] and ranged between 70 [14] and 72.5% [16] at 12 months. The latter results were similar compared with HC or MP [14,15,16]. In the other hand, resolution of symptoms after HeLP was statistically higher after HeLP versus RBL at 6 months (90% versus 53.3%, p < 0.001, respectively) [22]. Overall, symptomatic resolution ranged between 83.6% at 6 months [20] and 90.3% at 12 months [21]. Nevertheless, resolution rate was unavailable in three studies after LH [8, 11, 12] and in two studies after HeLP [9, 17].
Recurrence
After LH, two studies reported symptomatic recurrences, which ranged between 10 [16] and 11.3% [8] at 12 months. In the RCT by Poskus et al. [16], recurrence rates were significantly lower after HC, compared with LH and MP (0% versus 10% versus 22%, p < 0.004, respectively). No recurrence was reported after LH for three studies [11, 12, 15]. However, data on recurrence were unavailable for two studies [13, 14].
After HeLP, one study [17] reported a symptomatic recurrence rate of 8.3% at a mean of 5.8 months, and three other studies [9, 18, 19] reported recurrences from 5% at a median of 15 months to 9.4% at 12 months. Data on recurrence were unavailable for three studies [20,21,22].
Reoperation
Data on reoperation were available in three studies after LH [8, 14, 15] and in two studies after HeLP [19, 21]. In the study by Karahaliloglu et al. [8], 54.7% of patients were reoperated with redo LH, for insufficient treated nodes between 1 and 3 months. Two other studies [14, 15] reported no need for reoperation after LH, with a follow-up of 3 to 12 months. After HeLP, up to 7.8% of cases were reoperated within 5 months [19], with redo HeLP, RBL, transanal hemorrhoidal dearterialization, stapled hemorrhoidopexy, or HC [19, 21].
Discussion
The present systematic review included 14 studies describing LH [8, 11,12,13,14,15,16] and HeLP [9, 17,18,19,20,21,22], representing 1570 patients with grades I to IV HD. Primarily, laser therapies seemed to be safe. The single intraoperative complication was bleeding (0–1.9% for LH, 5.5–16.7% for HeLP), managed with intraoperative laser, suture, or conservatively. Postoperative complications occurred up to 64% of patients after LH and up to 23.3% after HeLP. However, these complications were frequently minor, which did not require therapy or were treated with conservative measures. Only 0.9% of postoperative complications required surgical therapy [8]. Moreover, 64% of bleeding reported after LH [12] consisted of post-defecatory bleeding, which did not require treatment and spontaneously resolved after the 7th postoperative day. Compared with other non-excisional therapy for HD, the RCT by Giamundo et al. [22] showed similar intraoperative bleeding rate associated with HeLP versus RBL (16.6% versus 10%, p = 0.12). Compared with excisional therapy, two RCTs [14, 15] showed lower intraoperative blood volume loss associated with LH versus HC. Moreover, postoperative complications were decreased with LH (urinary retention: 0% for LH versus 13.3% for HC, p = 0.038; anal stenosis: 0% for LH versus 13.3% for HC, p = 0.038) [15].
Secondarily, laser therapies are effective for the treatment of grades II and III HD, as shown by surgical indicators of postoperative outcomes. Resolution of symptoms ranged between 70 and 100% after LH and from 83.6 to 90% after HeLP. These rates are similar to the success rate of RBL reported in the literature. As shown by a retrospective study [23] of 750 patients with grades I to III HD treated with RBL, the success rate was 89%. Moreover, four RCTs included in our review reported similar resolution after LH compared with HC [14,15,16] or MP [16] and after HeLP compared with RBL [22]. Another surgical indicator was the recurrence rate, reported between 0 and 11.3% after LH and between 5 and 9.4% after HeLP. In the literature, recurrence rate at 12 months was reported up to 5% after mucopexy [24] and up to 11.1% [25] after hemorrhoidal artery ligation ± mucopexy. Compared with HC, LH showed conflicting results with one RCT reporting similar recurrence rate [15], but another RCT reporting decreased recurrences associated with HC [16]. Another surgical indicator of postoperative outcome was the reoperation rate, reported in 54% of patients after LH [8]. However, Karahaliloglu et al. [8] were the first to report their experience with LH, and this high reoperation rate seemed to decrease with progress in the learning curve. Moreover, two other studies reported no need for reoperation after LH, at 3 and 6 months of follow-up [14, 15]. Nevertheless, a long-term follow-up is mandatory to identify recurrences and the potential need for further intervention.
Thirdly, laser therapies conferred the advantages of a quick return to normal activities and low postoperative pain. The latter is explained by the absence of excision of tissue below the dentate line, where pain fibers are present [26]. Compared with HC, two RCTs showed decreased postoperative pain score associated with LH [15, 16]. Compared with RBL, another RCT showed decreased postoperative pain associated with HeLP [22]. However, pain comparison between studies is hazardous as postoperative analgesia varied significantly among studies.
The main limitation of the study is the heterogeneity of included studies. Perioperative characteristics, such as preoperative enema, antibioprophylaxis, anesthesia, and laser techniques, varied significantly among studies. Moreover, while grades II and III HD are good candidates for laser therapies, some studies included grades I and IV HD [8, 11, 20, 21]. Another weakness is the small population size of included studies, with the largest cohort composed of 341 patients [11]. This resulted in a decreased statistical power. Moreover, rare complications may be unidentified.
In this review, surgical indicators were used as surrogates of postoperative outcomes. Nevertheless, they were irregularly reported among studies. Moreover, Giamundo et al. [21] reported 9.7% of symptomatic persistence in the results section, but this was latter mentioned as persistence and/or recurrence in the discussion section. Inconsistency with outcomes definition precluded a meticulous analysis. As demonstrated by a recent systematic review [27], assessment of treatment efficiency should emphasize the use of validated scoring systems. However, none of the included study used these scores.
Overall, laser therapies appeared to be safe and effective techniques for the treatment of HD. Moreover, the learning curve is quick and was estimated from three to five cases [14]. These techniques could be alternatives to RBL or hemorrhoidal artery ligation ± mucopexy for the treatment of grade II or III HD. Only one RCT compared HeLP with RBL [21] and future research should focus on the comparison between laser and other non-excisional therapies of HD. Another unanswered question is the utility of the Doppler for the laser procedure. As reported by two RCTs [28, 29], the Doppler use did not show benefits for the hemorrhoidal artery ligation technique. Finally, benefits of LH or HeLP should be compared.
Conclusions
To conclude, non-excisional laser therapies, including LH and HeLP, are safe and effective. They should be considered for the treatment of grades II and III HD unresponsive to conservative management.
References
Johanson JF, Sonnenberg A (1990) The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 98(2):380–386
Aigner F, Bodner G, Conrad F, Mbaka G, Kreczy A, Fritsch H (2004) The superior rectal artery and its branching pattern with regard to its clinical influence on ligation techniques for internal hemorrhoids. Am J Surg 187(1):102–108
Goligher J (1980) Surgery of the anus, rectum and colon. 4th Ed Lond U K Balliere Tindall
van Tol RR, Kleijnen J, Watson AJM, Jongen J, Altomare DF, Qvist N et al (2020) European society of ColoProctology: guideline for haemorrhoidal disease. Color Dis
Milligan ETC, Naunton Morgan C, Jones LE, Officer R (1937) Surgical anatomy of the anal canal and the operative treatment of haemorrhoids. Lancet. 230(5959):1119–1124
Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR (2018) The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of hemorrhoids. Dis Colon Rectum 61(3):284–292
Barr LL, Jantz TA (1998) Effects of various laser wavelengths and energy levels on pig rectal submucosal tissue. J Laparoendosc Adv Surg Tech A 8(2):83–87
Karahaliloglu A (2007) First results after laser obliteration of first- and second-degree hemorrhoids. Coloproctology. 29:327–336
Salfi R (2009) A new technique for ambulatory hemorrhoidal treatment. Coloproctology 31(2):99–103
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7)
Jahanshahi A, Mashhadizadeh E, Sarmast M-H (2012) Diode laser for treatment of symptomatic hemorrhoid: a short term clinical result of a mini invasive treatment, and one year follow up. Pol Przegl Chir 84(7):329–332
Brusciano L, Gambardella C, Terracciano G, Gualtieri G, Schiano di Visconte M, Tolone S et al (2019) Postoperative discomfort and pain in the management of hemorrhoidal disease: laser hemorrhoidoplasty, a minimal invasive treatment of symptomatic hemorrhoids. Updat Surg
Plapler H, Hage R, Duarte J, Lopes N, Masson I, Cazarini C et al (2009) A new method for hemorrhoid surgery: intrahemorrhoidal diode laser, does it work? Photomed Laser Surg 27(5):819–823
Naderan M, Shoar S, Nazari M, Elsayed A, Mahmoodzadeh H, Khorgami Z (2017) A randomized controlled trial comparing laser intra-hemorrhoidal coagulation and Milligan-Morgan hemorrhoidectomy. J Investig Surg Off J Acad Surg Res 30(5):325–331
Alsisy A, Alkhateep YM, Salem IA (2019) Comparative study between intrahemorrhoidal diode laser treatment and Milligan–Morgan hemorrhoidectomy. Menoufia Med J 32(2):560–565
Poskus T, Danys D, Makunaite G, Mainelis A, Mikalauskas S, Poskus E et al (2020) Results of the double-blind randomized controlled trial comparing laser hemorrhoidoplasty with sutured mucopexy and excisional hemorrhoidectomy. Int J Color Dis 35(3):481–490
Giamundo P, Cecchetti W, Esercizio L, Fantino G, Geraci M, Lombezzi R et al (2011) Doppler-guided hemorrhoidal laser procedure for the treatment of symptomatic hemorrhoids: experimental background and short-term clinical results of a new mini-invasive treatment. Surg Endosc 25(5):1369–1375
Crea N, Pata G, Lippa M, Chiesa D, Gregorini ME, Gandolfi P (2014) Hemorrhoidal laser procedure: short- and long-term results from a prospective study. Am J Surg 208(1):21–25
De Nardi P, Tamburini AM, Gazzetta PG, Lemma M, Pascariello A, Asteria CR (2016) Hemorrhoid laser procedure for second- and third-degree hemorrhoids: results from a multicenter prospective study. Tech Coloproctol 20(7):455–459
Boarini P, Boarini LR, Boarini MR, Lima EM, Candelaria PA (2017) Hemorrhoidal laser procedure (HeLP): a painless treatment for hemorrhoids. J Inflamm Bowel Dis Disord 2(2)
Giamundo P, Braini A, Calabro’ G, Crea N, De Nardi P, Fabiano F et al (2018) Doppler-guided hemorrhoidal dearterialization with laser (HeLP): a prospective analysis of data from a multicenter trial. Tech Coloproctol 22(8):635–643
Giamundo P, Salfi R, Geraci M, Tibaldi L, Murru L, Valente M (2011) The hemorrhoid laser procedure technique vs rubber band ligation: a randomized trial comparing 2 mini-invasive treatments for second- and third-degree hemorrhoids. Dis Colon Rectum 54(6):693–698
Nakeeb AME, Fikry AA, Omar WH, Fouda EM, Metwally TAE, Ghazy HE et al (2008) Rubber band ligation for 750 cases of symptomatic hemorrhoids out of 2200 cases. World J Gastroenterol: WJG 14(42):6525–6530
Aigner F, Kronberger I, Oberwalder M, Loizides A, Ulmer H, Gruber L et al (2016) Doppler-guided haemorrhoidal artery ligation with suture mucopexy compared with suture mucopexy alone for the treatment of grade III haemorrhoids: a prospective randomized controlled trial. Colorectal Dis Off J Assoc Coloproctology G B Irel 18(7):710–716
Zhai M, Zhang Y-A, Wang Z-Y, Sun J-H, Wen J, Zhang Q et al (2016) A randomized controlled trial comparing suture-fixation mucopexy and Doppler-guided hemorrhoidal artery ligation in patients with grade III hemorrhoids. Gastroenterol Res Pract
Sanchez C, Chinn BT (2011) Hemorrhoids. Clin Colon Rectal Surg 24(1):5–13
Longchamp G, Liot É, Meyer J, Longchamp A, Toso C, Buchs NC et al (2020) Scoring systems as outcomes assessment of the treatments for haemorrhoidal disease: a systematic review of the literature. Int J Color Dis 7
Schuurman J-P, Borel Rinkes IHM, Go PMNYH (2012) Hemorrhoidal artery ligation procedure with or without Doppler transducer in grade II and III hemorrhoidal disease: a blinded randomized clinical trial. Ann Surg 255(5):840–845
Gupta PJ, Kalaskar S, Taori S, Heda PS (2011) Doppler-guided hemorrhoidal artery ligation does not offer any advantage over suture ligation of grade 3 symptomatic hemorrhoids. Tech Coloproctol 15(4):439–444
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Contributions
GL and EL conceived and designed the study. GL and EL acquired the data. GL, EL, JM, CT, NB, and FR interpreted the data. GL, EL, JM, CT, NB, and FR contributed to the writing of the manuscript and to its critical revision. GL, EL, JM, CT, NB and FR approved the final version of the manuscript.
the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Longchamp, G., Liot, E., Meyer, J. et al. Non-excisional laser therapies for hemorrhoidal disease: a systematic review of the literature. Lasers Med Sci 36, 485–496 (2021). https://doi.org/10.1007/s10103-020-03142-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03142-8