Abstract
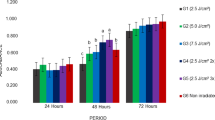
This study aimed to analyze the effects of laser irradiation on the membrane integrity and viability of stem cells from human exfoliated deciduous teeth (SHED) that were kept in serum starvation. Nutritional deficit was used to mimic the cellular stress conditions of SHED isolation for regenerative dental approaches, where laser therapy could be beneficial. SHED were cultured under serum starvation (MEMα + 1%FBS) for 1 or 24 h pre-irradiation (protocols A and B, respectively). Then, cells received low-level laser therapy (LLLT; 660 nm) at 2.5 J/cm2 (0.10 W; groups I and V), 5.0 J/cm2 (0.20 W; groups II and VI), 7.5 J/cm2 (0.30 W; groups III and VII), or remained non-irradiated (groups IV and VIII). During irradiation, cells were maintained in 1% FBS (groups I–IV) or 10% FBS (normal culture conditions; groups V–VIII). Membrane integrity was evaluated by quantifying lactate dehydrogenase (LDH) release (immediately after irradiation), and cell viability was assessed by the MTT assay (24, 48, and 72 h post-irradiation). Serum starvation did not alter LDH release by non-irradiated SHED, while LDH release decreased significantly in groups irradiated in 1% FBS (I and III), but not in groups irradiated in 10% FBS (V–VII), regardless the pre-irradiation conditions (protocols A/B). Cell viability was significantly higher 24 h after irradiation, in most protocol A groups. In contrast, cell viability remained mostly unaltered in protocol B groups. LLLT contributed to maintain membrane integrity in SHED subjected to nutritional deficit before and during irradiation with 0.10 or 0.30 W. Short serum starvation before irradiation improved SHED viability at 24 h post-irradiation.




Similar content being viewed by others
References
Carroll JD et al (2014) Developments in low level light therapy (LLLT) for dentistry. Dent Mater 30:465–475
Alghamdi KM, Kumar A, Moussa NA (2012) Low level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249
Tagliani MM et al (2010) Nutritional stress enhances cell viability of odontoblast-like cells subjected to low level laser irradiation. Laser Phys Lett 7:247–251
Nara Y, Matono S, Morioka T (1991) Regulatory action of low intensity laser on mitogenesis of cultured lymphocytes using concanavalin. Lasers Surg Med 3:293–298
Steinlechner CWB, Dyson M (1993) The effects of low level laser therapy on the proliferation of keratinocytes. Laser Ther 5:65–73
Souza LM et al (2018) Effect of low-level laser therapy on viability and proliferation of stem cells from exfoliated deciduous teeth under different nutritional conditions. Laser Phys 28:1–5
Ginani F et al (2015) Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci 30:2189–2194
Miura M et al (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812
Castro-Silva IL et al (2009) Preliminary analysis of the influence of low intensity laser (GaAlAs) in proliferation of human deciduous dental pulp derived cells. Innov Implant J Biomater Esthet 4:48–52
Fernandes AP et al (2016) Effects of low-level laser therapy on stem cells from human exfoliated deciduous teeth. J Appl Oral Sci 24:332–337
Zaccara IM et al (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30:2259–2264
Peplow PV, Chung TY, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29:285–304
Moura-Neto C et al (2016) Low-intensity laser phototherapy enhances the proliferation of dental pulp stem cells under nutritional deficiency. Braz Oral Res 30:1
Marques NCT et al (2017) Effects of PBM in different energy densities and irradiance on maintaining cell viability and proliferation of pulp fibroblasts from human primary teeth. Lasers Med Sci 32:1621–1628
Volpato LER et al (2011) Viability of fibroblasts cultured under nutritional stress irradiated with red laser, infrared laser, and red light-emitting diode. J Biomed Opt 16:075004
Azevedo LH et al (2006) Influence of different power densities of LILT on cultured human fibroblast growth. A pilot study. Lasers Med Sci 21:86–89
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Almeida-Lopes L et al (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Eduardo FP et al (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40:433–438
Oliveira CF et al (2011) In vitro effect of low-level laser on odontoblast-like cells. Laser Phys Lett 8:155–163
Pourzarandian A, Watanabe H, Ruwanpura SMPM, Aoki A, Ishikawa I (2005) Effect of low-level irradiation on culture human gingival fibroblasts. J Periodontol 76:187–193
Pereira NA et al (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers SurgMed 31:263–267
Ferreira MPP et al (2009) Effect of low-energy gallium-aluminum-arsenide and aluminium gallium indium phosphide laser irradiation on the viability of c2c12 myoblasts in a muscle injury mod. Photomed Laser Surg 27:901–906
Marques MM et al (2004) Effect of low power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34:260–265
Drent M et al (1996) Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 9:1736–1742
Yu L et al (2017) Antioxidant and antitumor activities of Capparis spinosa L. and the related mechanisms. Oncol Rep 37:357–367
Sumanasekera WK et al (2014) Cigarette smoke adversely affects functions and cell membrane integrity in c-kit+ cardiac stem cells. Cell Biol Toxicol 30:113–125
Basso FG et al (2012) In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent 2012:719452
Saito M et al (1997) Single column high—performance liquid chromatographic—fluorescence detection of immature, mature and senescent cross-links of collagen. Anal Biochem 253:26–32
Wagner VP et al (2013) Influence of different energy densities of laser phototherapy on oral wound healing. J Biomed Opt 18:28002
Marques NCT et al (2015) Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Med Sci 30:1815–1822
Silva LM et al (2016) Photobiomodulation protects and promotes differentiation of C2C12 myoblast cells exposed to snake venom. PLoS One 11:e0152890
Funding
This study was financially supported by Minas Gerais Research Foundation (FAPEMIG, Brazil, no. APQ-04004-16) and Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil, no. 88881.068437/2014–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted after approval by the Ethics Committee of Institute of Science and Technology, São Paulo State University (under protocol 46420).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
da Silva, P.C.S., Marques, N.P., Farina, M.T. et al. Laser treatment contributes to maintain membrane integrity in stem cells from human exfoliated deciduous teeth (shed) under nutritional deficit. Lasers Med Sci 34, 15–21 (2019). https://doi.org/10.1007/s10103-018-2574-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2574-x