Abstract
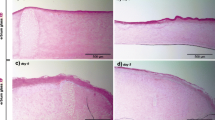
The molecular changes in gene expression following ablative laser treatment of skin lesions, such as atrophic scars and UV-damaged skin, are not completely understood. A standardized in vitro model of human skin, to study the effects of laser treatment on human skin, has been recently developed. Therefore, the aim of the investigation was to examine morphological and molecular changes caused by fractional ablative erbium:YAG laser treatment on an in vitro full-thickness 3D standardized organotypic model of human skin. A fractional ablative erbium:YAG laser was used to irradiate organotypic human 3D models. Laser treatments were performed at four different settings using a variety of stacked pulses with similar cumulative total energy fluence (60 J/cm2). Specimens were harvested at specified time points and real-time PCR (qRT-PCR) and microarray studies were performed. Frozen sections were examined histologically. Three days after erbium:YAG laser treatment, a significantly increased mRNA expression of matrix metalloproteinases and their inhibitors (MMP1, MMP2, MMP3, TIMP1, and TIMP2), chemokines (CXCL1, CXCL2, CXCL5, and CXCL6), and cytokines such as IL6, IL8, and IL24 could be detected. qRT-PCR studies confirmed the enhanced mRNA expression of IL6, IL8, IL24, CXCLs, and MMPs. In contrast, the mRNA expression of epidermal differentiation markers, such as keratin-associated protein 4, filaggrin, filaggrin 2, and loricrin, and antimicrobial peptides (S100A7A, S100A9, and S100A12) as well as CASP14, DSG2, IL18, and IL36β was reduced. Four different settings with similar cumulative doses have been tested (N10%, C10%, E10%, and W25%). These laser treatments resulted in different morphological changes and effects on gene regulations. Longer pulse durations (1000 μs) especially had the strongest impact on gene expression and resulted in an upregulation of genes, such as collagen-1A2, collagen-5A2, and collagen-6A2, as well as FGF2. Histologically, all treatment settings resulted in a complete regeneration of the epidermis 3 days after irradiation. Fractional ablative erbium:YAG laser treatment with a pulse stacking technique resulted in histological alterations and shifts in the expression of various genes related to epidermal differentiation, inflammation, and dermal remodeling depending on the treatment setting applied. A standardized in vitro 3D model of human skin proved to be a useful tool for exploring the effects of various laser settings both on skin morphology and gene expression during wound healing. It provides novel data on the gene expression and microscopic architecture of the exposed skin. This may enhance our understanding of laser treatment at a molecular level.





Similar content being viewed by others
References
Neis MM, Wendel A, Wiederholt T, Marquardt Y, Joussen S, Baron JM, Merk HF (2010) Expression and induction of cytochrome p450 isoenzymes in human skin equivalents. Skin Pharmacol Physiol 23:29–39
Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, Luscher B, Baron JM (2012) IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 129:426–433
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69–70:1–18
Mathes SH, Ruffner H, Graf-Hausner U (2014) The use of skin models in drug development. Adv Drug Deliv Rev 69–70:81–102
Marquardt Y, Amann PM, Heise R, Czaja K, Steiner T, Merk HF, Skazik‐Voogt C, Baron JM (2015) Characterization of a novel standardized human three‐dimensional skin wound healing model using non‐sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med 47:257–265
Sardana K, Manjhi M, Garg VK, Sagar V (2014) Which type of atrophic acne scar (ice‐pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg 40:288–300
Lee SJ, Kang JM, Chung WS, Kim YK, Kim HS (2014) Ablative non-fractional lasers for atrophic facial acne scars: a new modality of erbium:YAG laser resurfacing in Asians. Lasers Med Sci 29:615–619
Latowsky BC, Abbasi N, Dover JS, Arndt KA, Kaminer MS, Rohrer TE, Macgregor JL, Wesley NO, Durfee MA, Tahan SR (2012) A randomized, controlled trial of four ablative fractionated lasers for photoaging: a quadrant study. Dermatol Surg 38:1477–1489
Choi SH, Kim KH, Song KH (2015) Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol 29:1598–1605
Ko DY, Jeon SY, Kim KH, Song KH (2014) Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol 28:1529–1539
Orringer JS, Rittié L, Hamilton T, Karimipour DJ, Voorhees JJ, Fisher GJ (2011) Intraepidermal erbium:YAG laser resurfacing: impact on the dermal matrix. J Am Acad Dermatol 64(1):119–128
Helbig D, Paasch U (2011) Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Skin Res Technol 17:119–128
Orringer JS, Rittie L, Baker D, Voorhees JJ, Fisher G (2010) Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol 163:757–768
Amann PM, Marquardt Y, Steiner T, Hölzle F, Skazik-Voogt C, Heise R, Baron JM (2016) Effects of non-ablative fractional erbium glass laser treatment on gene regulation in human three-dimensional skin models. Lasers Med Sci 31(3):397–404
Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Investig Dermatol 115(6):1148–1151
Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W (2011) Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Investig Dermatol 131(11):2233–2241
Hattori F, Kiatsurayanon C, Okumura K, Ogawa H, Ikeda S, Okamoto K, Niyonsaba F (2014) The antimicrobial protein S100A7/psoriasin enhances the expression of keratinocyte differentiation markers and strengthens the skin’s tight junction barrier. Br J Dermatol 171:742–753
Gauglitz GG, Bureik D, Zwicker S, Ruzicka T, Wolf R (2015) The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol Physiol 28:115–123
Hvid M, Johansen C, Deleuran B, Kemp K, Deleuran M, Vestergaard C (2011) Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines—a possible link between reduced skin barrier function and inflammation? Exp Dermatol 20(8):633–636
Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, Tredget EE (2011) Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 226:1265–1273
Sideek MA, Teia A, Kopecki Z, Cowin AJ, Gibson MA (2016) Co-localization of LTBP-2 with FGF-2 in fibrotic human keloid and hypertrophic scar. J Mol Histol 47(1):35–45
Mofikoya BO, Adeyemo WL, Ugburo AO (2012) An overview of biological basis of pathologic scarring. Niger Postgrad Med J 19:40–45
Shah JM, Omar E, Pai DR, Sood S (2012) Cellular events and biomarkers of wound healing. Indian J Plast Surg 45:220–228
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40:1334–1347
Bullard KM, Mudgett J, Scheuenstuhl H, Hunt TK, Banda MJ (1999) Stromelysin-1-deficient fibroblasts display impaired contraction in vitro. J Surg Res 84:31–34
Qu L, Liu A, Zhou L, He C, Grossman PH, Moy RL, Mi QS, Ozog D (2012) Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 44:517–524
Ozog DM, Liu A, Chaffins ML, Ormsby AH, Fincher EF, Chipps LK, Mi QS, Grossman PH, Pui JC, Moy RL (2013) Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol 149:50–57
Melerzanov A, Lavrov A, Sakania L, Korsunskaya I, Petersen E, Sobolev V (2014) Effects of laser radiation on MMP gene expression in keratinocytes. Prime J 4:39
Zeitter S, Sikora Z, Jahn S, Stahl F, Strauß S, Lazaridis A, Reimers K, Vogt PM, Aust MC (2014) Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns 40(5):966–973
Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten O-W, Price A, Schechtman L, Stacey G, Stokes W (2005) Guidance on good cell culture practice. Altern Lab Anim 33:261–287
Hartung T (2007) Food for thought … on cell culture. Altern Anim Exp 24:143–147
Coecke S, Ahr H, Blaauboer BJ, Bremer S, Casati S, Castell J, Combes R, Corvi R, Crespi CL, Cunningham ML (2006) Metabolism: a bottleneck in in vitro toxicological test development. Altern Lab Anim 34:49–84
Sasaki G, Travis HM, Tucker B (2009) Fractional CO2 laser resurfacing of photoaged facial and non-facial skin: histologic and clinical results and side effects. J Cosmet Laser Ther 11:190–201
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746 (Review)
Ferraq Y, Black DR, Theunis J, Mordon S (2012) Superficial wounding model for epidermal barrier repair studies: comparison of erbium:YAG laser and the suction blister method. Lasers Surg Med 44(7):525–532
Kottner J, Hillmann K, Fimmel S, Seité S, Blume-Peytavi U (2013) Characterisation of epidermal regeneration in vivo: a 60-day follow-up study. J Wound Care 22(8):395–400
Wiederholt T, Heise R, Skazik C, Marquardt Y, Joussen S, Erdmann K, Schröder H, Merk HF, Baron JM (2009) Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp Dermatol 18(11):969–978
Heise R, Skazik C, Marquardt Y, Czaja K, Sebastian K, Kurschat P, Gan L, Denecke B, Ekanayake-Bohlig S, Wilhelm KP, Merk HF, Baron JM (2012) Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol 25:241–248
Acknowledgements
These studies were supported in part by a grant from Asclepion Laser Technologies, Jena, Germany.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding source
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the ethical committee of the University Hospital, RWTH Aachen, Germany.
Informed consent
A written informed consent was obtained from all participants/participating parents.
Rights and permissions
About this article
Cite this article
Schmitt, L., Amann, P.M., Marquardt, Y. et al. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med Sci 32, 805–814 (2017). https://doi.org/10.1007/s10103-017-2175-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2175-0