Abstract
Background/aim
Enterococcus faecalis and Enterococcus faecium cause human infections including bacteraemia and infective endocarditis (IE). Only few studies describing non-faecalis and non-faecium Enterococcus (NFE) infections have been conducted. We aimed to describe the incidence, prognosis, and focus of infection of bacteraemia with NFE.
Methods
This retrospective population-based study included all episodes of patients having a blood culture with growth of NFE between 2012 and 2019 in Region Skåne, Sweden. Information was collected from medical records. Episodes of bacteraemia caused by NFE were compared to episodes of bacteraemia caused by E. faecalis and E. faecium.
Results
During the study period, 136 episodes with NFE bacteraemia were identified corresponding to an incidence of NFE bacteraemia of 16 cases per 1,000,000 person-years among adults. Enterococcus casseliflavus (n=45), Enterococcus gallinarum (n=34), and Enterococcus avium (n=29) were the most common species. The most common foci of infection were biliary tract infections (n=17) followed by gastrointestinal infections (n=7). Urinary tract infections were not commonly caused by NFE (n=1), and no episodes of IE were caused by NFE. Polymicrobial bacteraemia was more common with NFE (73%) than with E. faecalis (35%) and E. faecium (42%). Community acquired infections were more common in bacteraemia with NFE compared to E. faecium. 30- and 90-day survival rates were 76% and 68%, respectively, and recurrent NFE bacteraemia was seen after 3% of the episodes.
Conclusion
Bacteraemia caused by NFE is rare and is often polymicrobial. Biliary tract focus is common in NFE bacteraemia whereas IE and urinary tract focus are uncommon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enterococcus is a genus of bacteria known to be commensals of the human gastrointestinal tract and they can occasionally cause severe infections, especially in health care-associated and in nosocomial settings [1,2,3]. Enterococci can cause a variety of conditions that can be complicated by bacteraemia, such as urinary tract infection, intra-abdominal infections, and infective endocarditis (IE) [4]. The majority of enterococcal infections are caused by the two species Enterococcus faecalis and Enterococcus faecium, with E. faecalis being the most common cause of human infections [1,2,3]. Other Enterococcus spp. known to cause human infections include Enterococcus casseliflavus, Enterococcus gallinarum, Enterococcus avium, Enterococcus durans, and Enterococcus hirae [5].
About 90% of the cases of enterococcal IE are caused by E. faecalis, and E. faecium is responsible for most of the remaining cases [6]. The proportion of patients with enterococcal bacteraemia that have IE has been estimated to be between 10 and 30% [7,8,9,10,11].
Bacteraemia caused by different Enterococcus species has not been thoroughly investigated, and [12] bacteraemia caused by uncommon enterococci is even less studied. Most of the reports are small case studies such as the one conducted in 2004 by Choi et al. [13] focusing on bacteraemia caused by E. casseliflavus and E. gallinarum. The first large-scale study with focus on non-faecalis and non-faecium Enterococcus species (NFE) described 186 episodes of bacteraemia in Taiwan and was presented 2010 by Tan et al. [14]. Tan et al. reported that the most common NFE in bacteraemia were E. casseliflavus, E. gallinarum, and E. avium with the most common types of infection being catheter-associated bloodstream infection, biliary tract infections, and intra-abdominal infections [14].
The aim of this study was to conduct a large retrospective population-based study of all patients with NFE bacteraemia between years 2012-2019 in the Region of Skåne, Sweden, to increase the knowledge on infections caused by these uncommon Enterococcus species, to allow for better future work-up and treatment.
Materials and methods
Study design and participants
This is a descriptive population-based retrospective study of patients with bacteraemia caused by NFE from 2012 to 2019 in the Region Skåne, Sweden. Information on all episodes with positive blood cultures for Enterococcus spp. from January 2012 to December 2019 was obtained from the Department of Clinical Microbiology database of Region Skåne, Sweden. The Department of Clinical Microbiology is the only laboratory in the region which had 1 million inhabitants 18 years and older (December 2012) at the beginning of the study period and 1.08 million (December 2019) at the end [15].
All blood cultures with growth of NFE were included and analysed. From the patients with blood cultures positive for E. faecalis and E. faecium, a random sample of 100 patients for each species was included for the analysis. The number of episodes in these comparison groups was chosen so that they were more than double the number of episodes in the largest NFE group. The sampling was done by choosing the first episode with a blood culture positive for E. faecalis and E. faecium every month leading to a total of 96 patients. The remaining 4 patients were randomly chosen from the end of June years 2013, 2015, 2017, and 2019. All the episodes of the patients with E. faecalis and E. faecium bacteraemia were included in the analysis thus leading to more episodes than total number of included patients.
Patients 18 years of age and over were included in the study. Episodes with incomplete medical history due to transfer to another hospital for treatment were excluded due to lack of information. Multiple positive blood cultures from the same patient were considered as one episode if obtained during the same hospitalisation or 14 days after being hospitalised; otherwise, they were counted as a new episode. One patient can thus contribute with more than one episode.
Microbiological analysis
A blood culture usually consisted of a set with one aerobic and one anaerobic culture vial. From 2012 to the end of 2014, the blood culture system BacT/ALERT® (bioMérieux, Inc., Durham, NC, USA) was used; from 2015 this system was replaced by BACTEX™ FX (BectonDickinson, Franklin Lakes, NJ, USA). The bacteria were identified using Matrix-Assisted Laser Desorption/Ionisation Time of Flight Mass Spectrometry (MALDI-TOF MS) with the database MBT Compass Library (Bruker Daltonics, Bremen, Germany). A score of 2.0 or higher was required for species identification.
The antimicrobial susceptibility testing for commonly used antibiotics was done by the disc diffusion method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST), or by determining the minimum inhibitory concentration (MIC) by using gradient strips, Etests (bioMérieux, Marcy l’Etoile, France), on agar plates according to manufacturer’s instructions. The EUCAST clinical breakpoints for Enterococcus spp. were used to categorise the isolates as sensitive, intermediate/increased exposure, or resistant according to the valid breakpoint table at the end of the study period [16].
Microbiological parameters included in the analysis were number of blood cultures taken, number of positive blood cultures with enterococci, mono- or polymicrobial bacteraemia, other bacteria isolated, and antimicrobial susceptibility for vancomycin and ampicillin.
Clinical data
Medical records were studied to obtain relevant patient demographics, medical background, and information on clinical course and outcomes. The collected information consisted of age, sex, comorbidities, number of episodes, microbiological findings, primary focus of infection, site of acquisition, variables included in criteria for IE [17, 18], radiological findings, findings from laboratory, and clinical assessments. At the time of positive blood culture, or within 24 hours from the positive blood culture, the severity of bacteraemia was assessed by calculating the Sequential Organ Failure Assessment (SOFA) score and using the definitions for sepsis as described in Sepsis-3 [19, 20].
To evaluate comorbidities, the Updated Charlson Comorbidity index was calculated [21]. The focus of infection was defined by fulfilment of at least two of the following criteria: (1) typical symptoms of focal infection, (2) isolation of the Enterococcus species in question at the site of infection, and (3) radiological signs of a focal infection, as described previously [10]. If less than two criteria were fulfilled, the episode was considered having an unknown focus of infection. Intraabdominal infections were divided into biliary tract infection (cholangitis, cholecystitis, and liver abscess) and gastrointestinal tract infection (gastrointestinal abscess, diverticulitis, appendicitis, ileitis, and post-abdominal surgery infection).
Surgical intervention was defined as any surgical procedure used to gain source control and treat the BSI. Complications to surgery that lead to BSI were not regarded as surgical procedures.
Site of acquisition was defined as nosocomial if a positive blood culture was drawn after 48 hours or more from hospital admission even if this could be due to a doctor’s delay in blood culturing. Health care-associated bacteraemia was defined according to Friedman et al. [22]. Episodes not fulfilling any of these criteria were regarded as community acquired. Modified Duke Criteria were used to assess the episode as definite, possible, or rejected IE [17, 18]. According to the criteria, an episode was regarded to constitute a major microbiology criterion only if there were two or more positive NFE blood cultures in a patient with non-nosocomial bacteraemia with unknown focus.
We also studied if the empiric antibiotic therapy was active against the blood culture isolate. Thus, piperacillin, ampicillin, vancomycin, or benzyl-penicillin or imipenem high doses (3g×3 and 1g×3 respectively) were regarded active against sensitive isolates whereas other empirical treatments were regarded as inactive. Time with antibiotic therapy, treatment at intensive care unit regarding admission and treatment with ventilator, vasopressors and dialysis, the need of surgery, length of stay, and 30- and 90-day survival.
Statistical data analysis
The data was analysed using IBM SPSS Statistics version 27. The number of inhabitants in the Region of Skåne, Sweden, during the study period was obtained from Statistic Sweden’s website [15]. The incidence was calculated by dividing the number of NFE cases with the mean number of inhabitants from the study period and expressed as number of cases per million inhabitants per year. The Shapiro-Wilk test was performed for all the variables to determine normal distribution, and data was described depending on normal distribution and scale type. Non-normally distributed variables with median and interquartile range (IQR) and categorical variables, for example binary variables, with amount (n) and percent (%). To determine statistical significances of differences between groups, chi-squared and 2-tailed Fisher’s exact tests were used for categorical variables and for non-parametric data Mann-Whitney U test. The comparison was made only between NFE, E. faecalis, and E. faecium; NFE were thus not compared with each other nor was E. faecalis compared with E. faecium. A value of p <0.05 was used to define statistical significance.
Ethics
The Regional Ethics Review Board in Lund, Sweden, granted an ethical approval for the study (2018-898).
Results
Epidemiology
Between 2012 and 2019, a total of 2263 episodes of blood cultures with Enterococcus species were recorded in the Region of Skåne, Sweden (Table 1). 137 episodes were NFE bacteraemia; one episode was excluded due to transfer to another hospital outside Region Skåne. The most common NFE episodes with bacteraemia were caused by E. casseliflavus (n=45), E. gallinarum (n=34), and E. avium (n=29), followed by E. hirae (n=10) and E. durans (n=9). The more uncommon NFE isolates (E. raffinosus, E. thailandicus, E. cecorum, and E. mundtii) were analysed as a group “Other Enterococcus spp.” (n=9). The incidence of NFE bacteraemia was 16 cases per 1,000,000 person-years among adults. Median age for all analysed cases was 74 years, and no significant age differences were found between the different species (Table 2). Bacteraemia with E. casseliflavus was significantly more common in females (53%) in comparison to E. faecalis (33%) and E. faecium (34%).
Microbiology and site of acquisition
Polymicrobial infections were significantly more common in bacteraemia with E. casseliflavus, E. gallinarum, and E. avium in comparison to both E. faecalis and E. faecium (Table 2). In bacteremia with all Enterococcus species, the most common site of acquisition was health care-associated (46%), but in bacteraemia caused by NFE community acquired infections were more common than in bacteraemia caused by E. faecalis and E. faecium (Table 2).
Focus of infection and infective endocarditis
Unknown focus was more common than known focal infection in NFE bacteraemia (69% versus 31%) (Table 3). The most common known foci of infection were biliary tract infection and gastrointestinal tract infection. E. avium was found to cause significantly more gastrointestinal infections in comparison to E. faecalis and E. faecium. E. casseliflavus, E. avium, and E. durans were significantly more common agents for biliary tract infections in comparison to E. faecalis. A significantly lower proportion of urinary tract infections were found in bacteraemia with E. casseliflavus, E. gallinarum, and E. avium in comparison to E. faecalis (Table 3).
Transthoracic echocardiography was performed in 112 patients, of whom 22 patients had NFE bacteraemia (16% of all NFE episodes), and transoesophageal echocardiography was performed in 41 patients of which 5 patients had NFE bacteraemia (4% of all NFE episodes). No episodes with IE caused by NFE were found (Table 3). IE was found in eight episodes of E. faecalis bacteraemia (6% of all episodes with E. faecalis) and in one episode of E. faecium bacteraemia (1%) (Table 3).
Antibiotic susceptibility
Most enterococci were sensitive to ampicillin apart from E. faecium where only 13% were sensitive (Table 4). One E. avium isolate and one E. faecium isolate were resistant to vancomycin (Table 4). Empiric therapy with effect against the isolate was given in 63% of episodes with NFE bacteraemia (Table 5).
Treatment, prognosis, and outcome
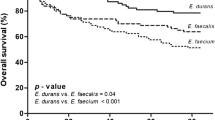
The median length of stay in hospital was 14 days for all the episodes with NFE, and episodes caused by E. casseliflavus, E. gallinarum, E. avium, and E. hirae had significantly shorter length of stay in comparison to E. faecium (Table 5). Fourteen percent of episodes with NFE required admittance to ICU (Table 5). Surgical intervention was needed in 43% of episodes (Table 5). The most common procedures were endoscopic retrograde cholangiopancreatography, nephrostomies, and different types of surgical drainages. The median time with antibiotic treatment was 13 days (Table 5). The 30-day survival rate was 76%, and the 90-day survival rate was 68% (Table 5). Recurrence within 6 months from concluded antibiotic treatment occurred in 3% of episodes (Table 5). E. casseliflavus, E. gallinarum, and E. avium had significantly lower recurrence rates in comparison to E. faecalis (Table 5).
Discussion
This study demonstrates that NFE bacteraemia occurs with an incidence of 16 cases per 1,000,000 person-years in adults in our region and that E. casseliflavus, E. gallinarum, and E. avium are the most frequently encountered species. E. casseliflavus and E. gallinarum were also reported by Tan et al. to be the most common species in NFE [14]. The most common enterococcal blood isolates in our study were E. faecalis (62%) and E. faecium (32%) accounting for 94% of all episodes of enterococcal bacteraemia, and this is also in line with previous studies [24, 25]. The proportion of episodes of enterococcal bacteraemia caused by NFE was lower in our study (6%) than reported previously from Taiwan by Tan et al. [14] and from South-Korea by Choi et al. [13], but higher than reported from the USA by Patterson et al. [24] and from Canada by Billington et al. [25].
Bacteraemia with enterococci is often either health care-associated or nosocomial [12, 24, 26, 27]. We report that a relatively high proportion, 36%, of all episodes with NFE bacteraemia were community acquired which is similar to the proportion in previous reports [13, 14, 25, 28].
Previously reported common foci of infection for NFE are the biliary tract, intravascular catheters, and gastrointestinal tract [13, 14, 28]. It is worth noticing that urinary tract infections are rare with NFE in comparison to E. faecalis and E. faecium. The results of this study follow a similar pattern of biliary tract and gastrointestinal infections being the most common foci, while at the same time most of the episodes with NFE bacteraemia have unknown focus. This might be due to our strict criteria for a focal infection even though Tan et al. [14] used similar criteria based on clinical, microbiological, and radiological findings. A higher proportion of polymicrobial infections can also indicate that the primary focus of infection is intra-abdominal in many episodes of bacteraemia caused by NFE. It is worth noticing that, in this study, clinicians often identified a possible focus of infection based on their clinical evaluation and started therapy without performing detailed radiological investigations.
We did not find any episodes of NFE-caused IE. IE has been reported to occur with NFE bacteraemia in some studies [14, 28, 29]. Since echocardiography was only performed in a minority of patients with NFE bacteraemia we cannot exclude the possibility that cases of IE might have been missed. However, since relapses were uncommon, we find it unlikely that many cases of IE were missed. From our results and earlier studies, it can be concluded that IE does not commonly occur in NFE bacteraemia, and we find it reasonable to suggest echocardiography to be performed only in cases of NFE bacteraemia where a specific suspicion of IE is raised. Regarding IE caused by bacteraemia with E. faecalis we found a lower proportion (6%) than reported before [8,9,10,11,12, 29, 30]. IE caused by E. faecium was uncommon, as found also in previous work by Anderson et al., Lwin and Bannan, and by Pinholt et al. [9, 11, 12]. The recently updated Duke-ISCVID criteria do not include non-faecalis Enterococcus species as a typical cause of IE [23], and our results are compatible with the classification of E. faecalis as a typical IE-pathogen and the non-faecalis enterococci as non-typical pathogens.
Regarding ampicillin resistance, our findings are comparable to Choi et al. [13] for E. gallinarum but in our study all E. casseliflavus isolates were sensitive. Our data show that empiric therapy with effect against the isolate was applied only in around half of the episodes, which is similar as reported before by Souhail et al. [31], but much higher than that reported by Pinholt et al. [12].
We report a 30-day survival of 76% which is higher than most previous reports [14, 26]. Factors such as comorbidities, different routines, and antibiotic susceptibility might influence the risk of death but also the likelihood of drawing blood cultures from patients presenting with relatively mild symptoms of infection will affect the proportion of survivors in different studies.
Strengths of this study include that it is large and one of the most comprehensive studies focusing on bacteraemia caused by NFE. The population-based approach gives a low risk for selection bias. Weaknesses include the retrospective design which is a limitation leading to lack of some relevant clinical data and microbiological information. Another limitation is that only a random sample of E. faecalis and E. faecium was included in the analysis.
As the dataset was large and multiple statistical comparisons were performed, there is also a risk for a multiple comparison problem. We tried to lower this risk by only making comparisons between NFE and E. faecium and E. faecalis avoiding comparisons between episodes caused by different NFE species and between episodes caused by E. faecalis and E. faecium.
In conclusion, we show that NFE bacteraemia is rare, more often community acquired, and can be distinguished from bacteraemia with E. faecalis and E. faecium by a higher proportion of polymicrobial infections and a higher proportion of infectious foci in the biliary tract. Urinary tract infections leading to bacteraemia and IE with NFE are very rare.
Data availability
Anonymized data will be shared upon reasonable request.
References
García-Solache M, Rice LB (2019) The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev 32(2). https://doi.org/10.1128/CMR.00058-18
Fiore E, Van Tyne D, Gilmore MS (2019) Pathogenicity of enterococci. Microbiol Spectr 7:4. https://doi.org/10.1128/microbiolspec.GPP3-0053-2018
Murray BE (1990) The life and times of the Enterococcus. Clin Microbiol Rev 3(1):46–65. https://doi.org/10.1128/CMR.3.1.46
Agudelo Higuita NI, Huycke MM (2014) Enterococcal disease, epidemiology, and implications for treatment. In: Gilmore MS, Clewell DB, Ike Y, Shankar N (eds) Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts eye and ear infirmary, Boston
Jett BD, Huycke MM, Gilmore MS (1994) Virulence of enterococci. Clin Microbiol Rev 7(4):462–478. https://doi.org/10.1128/CMR.7.4.462
Megran DW (1992) Enterococcal endocarditis. Clin Infect Dis 15(1):63–71. https://doi.org/10.1093/clinids/15.1.63
Dahl A, Iversen K, Tonder N, Hoest N, Arpi M, Dalsgaard M et al (2019) Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol 74(2):193–201. https://doi.org/10.1016/j.jacc.2019.04.059
Østergaard L, Bruun NE, Voldstedlund M, Arpi M, Andersen C, Schønheyder HC et al (2019) Prevalence of infective endocarditis in patients with positive blood cultures: a Danish nationwide study. Eur Heart J 40(39):3237–3244. https://doi.org/10.1093/eurheartj/ehz327
Lwin N, Bannan A (2020) A retrospective observational study on enterococcal bacteraemia and endocarditis at a regional hospital in New South Wales, Australia. Infect Dis Health 25(4):245–252. https://doi.org/10.1016/j.idh.2020.05.004
Berge A, Krantz A, Östlund H, Nauclér P, Rasmussen M (2019) The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection 47(1):45–50. https://doi.org/10.1007/s15010-018-1208-3
Anderson DJ, Murdoch DR, Sexton DJ, Reller LB, Stout JE, Cabell CH et al (2004) Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 32(2):72–77. https://doi.org/10.1007/s15010-004-2036-1
Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schønheyder HC, Gradel KO et al (2014) Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: a population-based cohort study. Clin Microbiol Infect 20(2):145–151. https://doi.org/10.1111/1469-0691.12236
Choi SH, Lee SO, Kim TH, Chung JW, Choo EJ, Kwak YG et al (2004) Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis 38(1):53–61. https://doi.org/10.1086/380452
Tan CK, Lai CC, Wang JY, Lin SH, Liao CH, Huang YT et al (2010) Bacteremia caused by non-faecalis and non-faecium enterococcus species at a Medical center in Taiwan, 2000 to 2008. J Infect 61(1):34–43. https://doi.org/10.1086/380452
Statistikmyndigheten SCB. Population by region, marital status, age and sex. Year 1968 - 2020 [Internet]. Stockholm: Statistiska centralbyrån; 2021 [updated 2021 Feb 22; cited 2021 Apr 1]. Available from: https://www.statistikdatabasen.scb.se/
EUCAST The European Comittee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 11.0, 2021 [Internet]. The European Comittee on Antimicrobial Susceptibility Testing; 2021 [updated 2021 Jan 1; cited 2021 May 10]. Available from: https://eucast.org/clinical_breakpoints/
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T et al (2000) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30(4):633–638. https://doi.org/10.1086/313753
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F et al (2015) 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36(44):3075–3128. https://doi.org/10.1093/eurheartj/ehv319
Lambden S, Laterre PF, Levy MM, Francois B (2019) The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 23(1):374. https://doi.org/10.1186/s13054-019-2663-7
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P et al (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173(6):676–682. https://doi.org/10.1093/aje/kwq433
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP et al (2002) Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797. https://doi.org/10.7326/0003-4819-137-10-200211190-00007
Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL et al (2023) The 2023 Duke-ISCVID Criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis:ciad271. https://doi.org/10.1093/cid/ciad271
Patterson JE, Sweeney AH, Simms M, Carley N, Mangi R, Sabetta J et al (1995) An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore) 74(4):191–200. https://doi.org/10.1097/00005792-199507000-00003
Billington EO, Phang SH, Gregson DB, Pitout JD, Ross T, Church DL et al (2014) Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 26:76–82. https://doi.org/10.1016/j.ijid.2014.02.012
Poh CH, Oh HM, Tan AL (2006) Epidemiology and clinical outcome of enterococcal bacteraemia in an acute care hospital. J Infect 52(5):383–386. https://doi.org/10.1016/j.jinf.2005.07.011
Maki DG, Agger WA (1988) Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 67(4):248–269
Reid KC, Cockerill IF, Patel R (2001) Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteremia: a report of 20 cases. Clin Infect Dis 32(11):1540–1546. https://doi.org/10.1086/320542
Olaison L, Kimmo S (2002) Enterococcal endocarditis in Sweden, 1995–1999: can shorter therapy with aminoglycosides be used? Clin Infect Dis 34(2):159–166. https://doi.org/10.1086/338233
Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS et al (2013) Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 8(12):e82665. https://doi.org/10.1371/journal.pone.0082665
Souhail B, Le Maréchal M, Manuello R, Chrétien R, Charlot P, Déroudilhes G et al (2019) Antibiotic therapy for Enterococcus bacteraemia: warning for the antimicrobial stewardship team. Eur J Clin Microbiol Infect Dis 38(11):2087–2095. https://doi.org/10.1007/s10096-019-03645-5
Acknowledgements
We would like to thank Mrs. Lena Hyllebusk for help with obtaining microbiological data and Mrs. Emma Söderdahl for help with obtaining medical records.
Funding
Open access funding provided by Lund University. This work was supported by the Swedish Government Fund for Clinical Research (ALF), the Foundations of Österlund and Skåne University Hospital, all to Magnus Rasmussen. None of the funding bodies had any influence on this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lohikoski, R., Oldberg, K. & Rasmussen, M. Bacteraemia caused by non-faecalis and non-faecium Enterococcus species—a retrospective study of incidence, focus of infection, and prognosis. Eur J Clin Microbiol Infect Dis 43, 45–53 (2024). https://doi.org/10.1007/s10096-023-04690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04690-x