Abstract
Rising healthcare complications due to fungal infections increase the importance of efficient specimen collection and maintenance systems for correct identification and diagnosis. The CLSI M40-A2 protocol provides guidelines for laboratories assessing quality of medical transport devices, including swab transport systems (STS). This study assessed the efficiency of the Sigma Transwab® foam and flock swab in recovering and maintaining viability of different Candida spp. including C. auris, in different test conditions. Both swab types recovered and maintained viability of all Candida spp. with greater CFU at room temperature after incubation (24 and 48 h) in comparison with swabs maintained at 4 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive fungal diseases are a major global health problem with mortality rates between 30 and 90% and a large proportion of these diseases caused by of Aspergillus, Cryptococcus, Candida, or Pneumocystis [1]. Candida spp. have been identified as a leading cause of pathogenic diseases, with candidiasis known to affect more than a quarter of a million patients worldwide annually [2]. This increase in opportunistic fungal pathogens can be linked to several factors including chemotherapy, transplant situations which result in an increase in immunocompromised patients [3]. One species of Candida that is of particular concern is C. auris. Since the first report of C. auris in 2009 [4], there have been concerns of rapid spread across the globe [5]; misidentification with other Candida spp. [6,7,8,9]; seriousness of associated infections including reports of isolation from the bloodstream, urinary tract, ear canal, wounds, heart muscle and bone [10]; high mortality rate, related to bloodstream infections [10,11,12]; and its antifungal resistance [6, 13].
Rapid and efficient isolation and identification of infectious agents are paramount to successful treatment. Medical transport devices, particularly swab transport systems (STS), have been used in clinical and laboratory diagnosis and are often used for their low cost, ease of use and ability to maintain microorganism viability over extended periods of time [14]. The Clinical and Laboratory Standards Institute (CLSI) M40-A2 is an approved standard which outlines testing procedures for liquid transport systems and provides manufacturers and end-users with a criteria for compliance [15]. This standard focuses on transport and collection devices and recovery of specimen, focuses largely on bacteria and only identifies yeasts for quality control of urine transport systems. Thus, it is important to assess and compare efficiency and effectiveness of swabs and collection devices in maintaining and recovering the integrity of pathogenic yeast specimen. In the study by Gizzie and Adukwu [14], the authors showed that both flock and foam Sigma Transwab®, swab types, were efficient at recovering and maintaining the recommended organisms in the M40-A2 standard including the difficult pathogen Neisseria gonorrhoea. The focus of this current study was to assess and compare these two swab types, Sigma Transwab® foam and flocked swab, for maintenance of viability and recovery of different Candida spp., including C. auris in vitro. This investigation is important as transport, maintenance and recovery of clinically important fungal pathogens are of relevance to medical and clinical laboratories and to ensure accuracy of diagnosis.
Methods
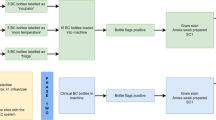
The CLSI M40-A2 protocol was used with some minor adaptations. The STS used in this study were both manufactured by Medical Wire and Equipment (MWE; Corsham, UK) and included their Sigma Transwab® foam (MW176S) and Sigma Transwab® Purflock® (MW176PF); both swab types are recommended for use for wound, skin and throat and utilise a liquid amies–carrying media. The MW176S employs a cellular foam bud, while the MW176PF employs a flocked fibre tip. Candida spp. used in this study include C. auris NCPF 8971, C. albicans NCPF 3179, C. tropicalis NCPF 3111, C. parapsilosis ATCC 22019 and C. glabrata ATCC 2001. Smears on microscope slides were produced for each species of Candida, and a Gramme stain was performed. Candida spp. stored on microbank beads at − 80 °C were grown on Sabouraud Dextrose Agar (SDA; EO Labs, Bonnybridge, UK) at 30 °C for 48 h and used to inoculate 0.85% physiological saline (OXOID, Basingstoke, UK) until turbidity reached McFarland Standard 0.5 (OD625nm 0.08–0.1) with a final working dilutions of 104 and 103 CFU/ml, with 100 μl aliquots dispensed in triplicates into a 96-well plate. The swabs were then immersed in the aliquots, and the dilutions were absorbed for a period of for 10 s after which the swabs were placed in the transport medium and maintained at either room temperature (RT) or 4 °C for 0, 24 and 48 h (T0/T24/T48). The swabs were removed from each well containing the swab/aliquot, rolled on the agar medium (SDA agar) and incubated at 30 °C for 48 h according to the CLSI M40-A2 standard. Following the incubation period, the colonies were enumerated and CFU values determined. The M40-A2 standard indicates that using the roll plate method, the enumerated counts should be ≥ 5 CFU after the specified holding period for specimen held at RT or at 4 °C, when the dilution is compared with the counts from T0 for the STS to be acceptable.
Results
Results from Gram staining procedure showed that all Candida spp. presented the Gram-positive appearance with typical round/oval budding morphology. There were no differences observed with the swab types in the shape and morphology of the test microorganism; however, we noticed that the C. tropicalis NCPF 3111 comprised budding yeast cells with some hyphal germ tubes and pseudohyphae. All strains of Candida, including C. auris, were successfully recovered by both foam, flocked swabs at room temperature and at 4 °C, and were found to be complaint with the M40-A2 criteria (Table 1). At time 0, there was no significant difference between the CFU between the Candida spp. recovered using both flock and foam swabs at room temperature and at 4 °C; however, after incubation for 24 h and 48 h, the CFU of the test organisms at room temperature was consistently greater than those grown at 4 °C (Fig. 1).
Enumeration of Candida auris on Sabouraud dextrose agar plates following maintenance at a Sigma Transwab® foam at T0; b Sigma Transwab® foam at T48 and RT; c Sigma Transwab® foam at T48 and 4 °C; d Sigma Transwab® Purflock® at T0; e Sigma Transwab® Purflock® at T48 and RT; and f Sigma Transwab® Purflock® at T48 and 4 °C
Discussion
Swab transport systems have been used in the maintenance and management of specimen. In clinical settings, these STSs aid accuracy and timeliness of diagnosis, which highlights their importance. The type and quality of swab is also important in enabling efficient management of specimen, hence the development of CLSI M40-A2 standard. Following the protocols set out by this standard, we investigated and compared the recovery of Candida spp. using the foam and flocked Sigma Transwab® swabs. The STS used in this study efficiently recovered and maintained the growth of Candida spp. including the yeast C. auris, which is of particular interest to clinicians due to misdiagnosis, link to nosocomial infections and drug resistance of this pathogen. While the recent study by Scansen et al. [16] and Gandhi et al. [17] demonstrated recovery of several Candida spp. and other pathogenic fungi using other commercial STS based on the M40-A2 protocol, they did not investigate recovery of C. auris. This study investigates the recovery and maintenance of different Candida spp. including C. auris using the M40-A2 protocol. The M40-A2 does not currently directly address the recovery of yeasts, with the exception of guidance for urine transport systems [15]. In this study, we also found that when using the protocol for adjusting initial inocula, enumeration of the yeasts was lower than that typically determined from bacteria cells, which is possibly as a result of the characteristic differences in sizes of bacteria and yeast; bacteria are smaller in size 0.2 to 2.0 μm in diameter and 2 to 8 μm in length, while the yeasts range between 2 and 60 μm [18, 19].
Of the Candida spp. used within this study, only C. auris is a known clinical isolate, and in future studies, we recommend using a panel of clinically pathogens which would better reflect the use of STS in clinical situations. However, using the reference strain cultures is in line with the M40-A2 protocol, which utilises quality control strains for testing STS. While the study by Scansen et al. [16] suggested that foam swabs were superior to flocked swabs particularly when used in antigen-testing experiments, in our study, we did not notice any significant differences in the recovery of the Candida spp. using both swab types. This study provides evidence of recovery of relevant yeasts including organisms of clinical importance such as the C. albicans and C. auris using the MWE Sigma Transwab® foam and flock swabs and offers clinical laboratories confidence to utilise these swabs in maintenance and transport of pathogenic and non-pathogenic yeasts.
References
Fisher MC, Gurr SJ, Cuomo CA, Blehert DS, Jin H, Stukenbrock EH, Stajich JE, Kahmann R, Boone C, Denning DW, Gow NAR, Klein BS, Kronstad JW, Sheppard DC, Taylor JW, Wright GD, Heitman J, Casadevall A, Cowen LE (2020) Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 11:e00449–e00420. https://doi.org/10.1128/mBio.00449-20
Arendrup MC, Patterson TF (2017) Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S445–S451. https://doi.org/10.1093/infdis/jix131
Vecchione A, Florio W, Celandroni F et al (2017) Comparative evaluation of six chromogenic media for presumptive yeast identification. J Clin Pathol 70:1074–1078. https://doi.org/10.1136/jclinpath-2017-204396
Satoh K, Makimura K, Hasumi Y et al (2009) Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. https://doi.org/10.1111/j.1348-0421.2008.00083.x
Lockhart SR, Etienne KA, Vallabhaneni S et al (2017) Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. https://doi.org/10.1093/cid/ciw691
Kathuria S, Singh PK, Sharma C et al (2015) Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol 53:1823–1830. https://doi.org/10.1128/JCM.00367-15
Lockhart SR, Jackson BR, Vallabhaneni S et al (2017) Thinking beyond the common Candida species: need for speciation of Candida due to the emergence of multidrug resistant Candida auris. J Clin Microbiol 55:JCM.01355–JCM.01317. https://doi.org/10.1128/JCM.01355-17
Lockhart SR, Berkow EL, Chow N et al (2017) Candida auris for the clinical microbiology laboratory: not your grandfather’s Candida species. Clin Microbiol Newsl 39:99–103. https://doi.org/10.1016/j.clinmicnews.2017.06.003
Kordalewska M (2017) Rapid and accurate molecular identification of the emerging multidrug-resistant pathogen. Candida auris 55:2445–2452
Sarma S, Upadhyay S (2017) Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resist 10:155–165. https://doi.org/10.2147/IDR.S116229
Tsay S, Kallen A, Jackson BR et al (2018) Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin Infect Dis 66:306–311. https://doi.org/10.1093/cid/cix744
Sears D, Schwartz BS (2017) Candida auris: an emerging multidrug-resistant pathogen. Int J Infect Dis 63:95–98. https://doi.org/10.1016/j.ijid.2017.08.017
Chowdhary A, Voss A, Meis JF (2016) Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J Hosp Infect 94:209–212. https://doi.org/10.1016/j.jhin.2016.08.004
Gizzie N, Adukwu E (2016) Evaluation of liquid-based swab transport systems against the new approved CLSI M40-A2 standard. J Clin Microbiol 54:1152–1156. https://doi.org/10.1128/JCM.03337-15
Clinical and Laboratory Standards Institute C. M40-A2: quality control of microbiological transport systems; Approved Standard - Second Edition. Clin Lab Stand Inst Published Online First:2014.https://clsi.org/media/1453/m40a2_sample.pdf (accessed 10 May 2018)
Scansen KA, Bonsu BK, Stoner E et al (2010) Comparison of polyurethane foam to nylon flocked swabs for collection of secretions from the anterior nares in performance of a rapid influenza virus antigen test in a pediatric emergency department. J Clin Microbiol 48:852–856. https://doi.org/10.1128/JCM.01897-09
Gandhi B, Summerbell R, Mazzulli T (2018) Evaluation of the Copan ESwab transport system for viability of pathogenic fungi by use of a modification of Clinical and Laboratory Standards Institute document M40-A2. J Clin Microbiol 2018:56(2)
Griffin DM (1985) A comparison of the roles of Bacteria and Fungi. In: Leadbetter ER, Poindexter JS (eds) Bacteria in nature, vol 1. Springer, Boston. https://doi.org/10.1007/978-1-4615-6511-6_8
Moore V.L. (2009) Microbiology basics. APIC Text of Infection Control & Epidemiology, 3rd edition. http://eta.health.usf.edu/publichealth/PHC6562/Midterm_Final_ExtraCredit_pools/HO1_Microbiology_Basics_APIC_Chapter16.pdf
Funding
This work was funded by Medical Wire and Equipment, Corsham, UK, and supported by the Faculty of Health and Applied Sciences, University of the West of England, Bristol, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elcocks, E., Adukwu, E. Laboratory evaluation of the Sigma Transwab® transport system for the recovery of Candida species using the Clinical and Laboratory Standards Institute (CLSI) document M40-A2. Eur J Clin Microbiol Infect Dis 40, 735–738 (2021). https://doi.org/10.1007/s10096-020-04062-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-04062-9