Abstract
The purpose of this study was to prospectively evaluate the impact of the use of L. plantarum I1001 applied vaginally on Vulvovaginal Candidiasis (VVC) time-until-recurrence after treatment with single-dose vaginal clotrimazole. This was a clinical open-label, prospective study of two non-randomized parallel cohorts with symptomatic acute VVC: (1) 33 sexually active women 18–50 years old, prescribed a standard single-dose 500 mg vaginal tablet of clotrimazole followed by vaginal tablets with L. plantarum I1001 as adjuvant therapy, and (2) 22 women of similar characteristics but prescribed single-dose clotrimazole only. Use of the probiotic and factors that might influence recurrence risk (age, recurrent VVC within previous year, antibiotic prior to study enrolment, diaphragm or IUD contraception, among others) were included in a multivariate Cox regression model to adjust for potential between-cohort differences. Probiotic use was associated with a three-fold reduction in the adjusted risk of recurrence (HR [95 %CI]: 0.30 [0.10–0.91]; P = 0.033). Adjusted free-survival recurrence was 72.83 % and 34.88 % for the probiotic and control groups, respectively. A higher cumulative recurrence was also observed in cases with use of antibiotics prior to enrolment (HR [95 %CI]: 10.46 [2.18–50.12]; P = 0.003). Similar findings were found at six months after azole treatment in women with RVVC. Overall, good compliance with the probiotic was reported for 91.3 % of women. The study suggests that follow-up therapy with vaginal tablets with L. plantarum I1001 could increase the effectiveness of single-dose 500 mg clotrimazole at preventing recurrence of VVC, an effect that was also observed in women with recurrent vulvovaginal candidiasis (RVVC) after six months of azole treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvovaginal candidiasis (VVC) is one of the most frequent infections of the female genital tract and is mainly associated with Candida albicans (followed by Candida glabrata) [1]. Candida species are normally found in the lower genital tract of 10–20 % of healthy women of childbearing age [2]. Evolution from colonization to symptomatic infection involves different host factors such as susceptibility and inflammatory responses and/or the imbalance of vaginal microbiota [3].
Available literature on VVC suggests that 75 % of women will show a VVC episode [4, 5] through their lifetime, and that 5–10 % of all women will experience recurrent vulvovaginal candidiasis (RVVC), i.e ≥4 episodes/year [6].
Short-term and single-dose vaginal antifungals are successful in 80–90 % of acute uncomplicated cases [7, 8]. However, azole resistance rates are above 15 % in women with RVVC [9, 10]. Antifungal resistance, infection recurrence and side effects of pharmacological treatments are key issues for patients and their physicians seeking alternative interventions for VVC.
Probiotics are defined as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” [11]. Probiotic properties are strain-specific so their positive health effects cannot be extrapolated to other strains of the same species or genus [12]. Lactobacilli are the predominant bacteria in a healthy vaginal ecosystem, and have been proposed for the treatment and prevention of genitourinary infections including candidiasis [4, 6]. Local use of these microorganisms results in immunomodulation responses and the restoration of vaginal microbiota, interfering with the colonization and growth of potential pathogens such as Candida [13]. A Lactobacilli-dominated vaginal microbiota produces significant levels of lactic acid with strong microbicidal properties [14].
Evidence of synergy between adjuvant local Lactobacilli and azole treatment for VVC has been reported [9, 15]. Nevertheless, evidence of the benefit of adding probiotics to the standard azole on the risk of symptomatic recurrence is lacking.
Recently, a pilot study for the evaluation of colonization and tolerability of a vaginal tablet of L. plantarum strain I1001 (deposit code CECT7504) in healthy women, reported that use of this formulation, three times a week on alternate days, achieves an adequate vaginal lactobacilli concentration [16]. These positive results led us to prospectively evaluate the impact of the use of L. plantarum I1001, applied vaginally, on VVC recurrence after a single-dose vaginal clotrimazole.
Patients and methods
A clinical open-label, prospective study of two non-randomized parallel cohorts was conducted in the out-patient gynaecological departments of the Instituto Palacios de Salud y Medicina de la Mujer and the Instituto de Medicina EGR (Madrid) from June 2013 until February 2015.
The first cohort (n = 33) corresponded to sexually active women between 18 and 50 years old with symptomatic acute VVC, who were prescribed with a standard single-dose 500 mg vaginal tablet of clotrimazole, followed by the L. plantarum I1001 vaginal probiotic tablet (Melagyn® Probiotico Vaginal, Gynea by Kern Pharma, Spain; each tablet contains L. plantarum I1001 at minimum 1 × 108 colony forming units) as adjuvant therapy (one tablet, three times a week on alternate days, for two consecutive months, excluding days with menstruation). The second cohort (n = 22) compromised women of similar characteristics but prescribed the single-dose clotrimazole treatment only.
Exclusion criteria (both cohorts) were pregnancy/breast feeding, delivery or abortion within the previous trimester, signs of other vaginal infections, abnormal genital bleeding within the previous semester, or any concomitant medication during follow-up that might significantly influence the evaluation and/or study results (including beta-lactams, clindamycin and tetracycline). Informed written consent was obtained from all participants prior to enrolment. All the study materials were approved by the Ethical Committee of the Hospital Universitario de la Princesa (Madrid, Spain).
Vaginal probiotic and dose administration
L. plantarum I1001 (CECT7504) is a selected, patented strain which has been tested in vitro, showing good adherence to vaginal epithelial cells (VEC), high acidification of simulated vaginal media, high tolerance to antimicrobial factors of inflamed vaginal fluid, and intrinsically resistant to high concentrations of some typical antibiotics (ATB) and antifungals used for vaginal affections. This L. plantarum I1001 formulation has been previously tested in healthy women during a pilot open label clinical trial at Hospital Vall d’Hebron (Barcelona, Spain) [16], showing successful colonization of the vagina for at least 48 hours, therefore, allowing its application on alternate days.
Data collection and follow-up
The primary outcome was the recurrence-free survival of signs/symptoms of VVC, as collected in the follow-up visits at two and three months after the azole treatment, within the possibility of a six-month visit for women with history of RVVC.
Specific risk factors (age, diabetes mellitus, RVVC within the last year, ATB prior to enrolment, diaphragm/intrauterine device or oral contraception, immunosuppression and history of other non-candida vulvovaginitis [VV]) were recorded at baseline. Self-reported presence of pruritus, vulvar soreness/irritation and burning vulvar pain, and signs of vaginal discharge, vulvar erythema and edema, and malodorousness, were assessed using a semi-quantitative scale (0 = absent, 1 = light, 2 = moderate and 3 = severe). In the L. plantarum I1001 cohort, physicians rated the effectiveness and tolerability of the product, using a semi-quantitative scale (0 = inadequate, 1 = fair, 2 = good, 3 = very good), while women also reported their opinion on the product and evaluated its tolerability and their satisfaction using the Spanish version of the Treatment Satisfaction Questionnaire for Medication (TSQM version 1.4) [17].
Statistical analysis
Baseline characteristics (including known risk factors for VVC) of both cohorts were compared with Fisher’s exact test (categorical variables) and Mann-Whitney’s U test (quantitative variables). Values were summarized and presented as percentages or mean ± standard deviation (SD). For the main clinical outcome (VVC recurrence-free survival), use of the vaginal probiotic and additional factors that might influence the risk of recurrence (see above) were included in a multivariate Cox (proportional-hazards) regression model; these results are presented as Hazard ratios (HR) and their 95 % confidence intervals (95 %CI). Drop-outs were censored at the time of last follow-up. In an attempt to assess the change in recurrence within cohorts, we compared the average number of VVC episodes per trimester within 12 months prior to enrolment including the baseline episode of women with three-months follow-up (obtained from clinical records) to those observed during the follow-up at three months, by using the non-parametric Wilcoxon signed-rank test. Only subjects with complete clinical record for the past 12 months and complete follow-up at three months were considered for this later analysis. Results on the TSQM are presented as median and interquartile range (IQR), because of their non-normality. No imputation was performed for missing values, and significance was set at a level of 5 %, two-tailed. All statistical tests were performed with SPSS Statistics for Windows v22.0 (IBM Corp. 2013. Armonk, NY).
Results
Baseline data
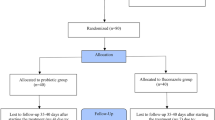
A general description of the patients and current VVC episode is presented in Table 1. Flow of patients throughout the study is presented in Fig. 1. Drop-out rate was slightly higher in the clotrimazole-only cohort (18.5 % vs. 5.7 % at 2 months, and 24.1 % vs. 15.4 % at 3 months), although the difference did not reach statistical significance.
Among the 55 women from both cohorts, 50 (91 %) had a history of previous VVC anytime during their lifespan (Table 1), from which 33 (63.5 %) met criteria for VVC within the 12 months prior to enrolment. The number of VVC episodes during the previous 12 months was higher in the L. plantarum I1001 cohort (4.55 ± 2.58 vs. 3.53 ± 1.88), although this difference did not reach statistical significance. Conversely, previous non-Candida VVs were reported exclusively in the antifungal-only cohort (n = 3, P = 0.059, statistical trend).
Current VVC was generally characterized by the specialist with vulvar erythema, vaginal discharge, edema and burning/soreness, without differences between cohorts regarding the number of patients with each symptom.
Patients’ and gynaecologists’ evaluation of the vaginal probiotic
Overall, patients treated with the vaginal probiotic reported a compliance of 91.3 %. The TSQM (n = 29) results showed that patients reported high ratings of efficacy, lack of side effects, convenience and overall satisfaction (Table 2). Only one patient reported a side effect (edema and erythema) of moderate intensity, and rated it as “somewhat affecting her satisfaction with the treatment” in the TSQM questionnaire (i.e. a score of 3 in a Likert 1–5 scale). Moreover, gynecologists considered efficacy of the test product in their patients (n = 31) as “good”/“very good” in 87 % of cases, and tolerability as “good”/“very good” in 100 % of them.
Time until symptomatic recurrence
Overall, 10/31 (32.2 %) of women in the probiotic group and 10/17 (58.8 %) of women in the control group had experienced one or more symptomatic recurrences during the 3–month follow-up (P = 0.125).
The Cox regression model on the combined cohorts (Table 3) showed that adjusted HR for developing a new VVC within three months of the single-dose clotrimazole, depending on probiotic use, was 0.30 (95 %CI: 0.10–0.91) and that this effect was statistically significant (P = 0.033, Fig. 2). Among other risk factors, ATB prior to enrolment also showed a significant effect, with an adjusted HR of 10.46 (95 %CI: 2.18–50.12); (P = 0.003, Fig. 3). Thus, comparison of the recurrence-free survival shows a clear increase in adjusted survival at 3 months (73 % vs. 35 %, i.e. a +108.6 % increase) in women receiving the probiotic, while the ‘previous ATB’ factor shows marked effect in the opposite direction (6 % vs. 68 %).
Recurrence-free survival curve 3 months after single-dose clotrimazole treatment. Comparison of the overall recurrence-free survival between patients treated with clotrimazole 500 mg single dose-only vs. clotrimazole + L. plantarum I1001. The difference on adjusted survival between the two cohorts is significant, with a higher recurrence-free survival in patients receiving adjuvant vaginal probiotic (HR [0.30 [95 %CI: 0.10–0.91]; P = 0.033, multivariate Cox regression model)
Recurrence-free survival curve 3 months after single-dose clotrimazole treatment. Patients with history of antibiotic treatment (ATB) prior to enrolment. Comparison of the overall recurrence-free survival depending on the use of antibiotic treatment prior to enrolment. The difference on adjusted survival between the two cohorts achieves significance with a markedly lower recurrence-free survival in patients with such risk factor (HR: 10.46 [95 %CI: 2.18–50.12]; P = 0.003, multivariate Cox regression model)
Analysis of the subgroup with history of RVVC (n = 23 of the clotrimazole + probiotic cohort, and n = 12 from the clotrimazole cohort) at 6-months of follow-up, showed a similar difference in adjusted survival among treatment cohorts (63 % vs. 22 %, Supplementary material). The adjusted HR for symptomatic recurrence depending on probiotic use was 0.30 (95 %CI: 0.10–0.89; P = 0.03), likewise for the effect of ‘previous ATB’ (Supplementary material).
Differences in the number of episodes
In the L. plantarum I1001 cohort the average number of VVC episodes per trimester during the 3-month follow-up was 0.45 ± 0.72, down from 1.14 ± 0.65 episodes/trimester during the 12 months prior to enrolment, thus representing a reduction of 59 % (P = 0.001). Conversely, such difference was not observed in the clotrimazole-only cohort (0.67 ± 0.62 vs. 0.88 ± 0.47 episodes/trimester; P > 0.1).
Discussion
In the present study, follow-up therapy with vaginal tablets with L. plantarum I1001 after a single-dose 500 mg clotrimazole was found to significantly reduce the risk of symptomatic recurrence within 3 months after an acute VVC, with similar findings in the subset of patients with RVVC within 6 months of follow-up.
Although most baseline characteristics were comparable, patient distribution among cohorts was not randomized and resulted in a larger number of the VVC episodes of women with any previous VVC in the last 12 months and a different history of non-Candida VV in the probiotic cohort. Taking into account the lack of randomization and those differences between cohorts, we considered appropriate to use a multivariate survival test to study the primary outcome (the recurrence-free survival). After adjustment for potential confounding factors by Cox multivariate analysis, the effect of adjuvant probiotic was significant in the full study population (at 3-months) and in women with RVVC within a 6-month follow-up. Nevertheless, we were surprised to observe that the hazard ratio was virtually the same in the full population at 3 months and in the subpopulation with RVVC at 6 months. However, this lack of difference may be due to the small study sample. In future studies, it would be interesting to reproduce the same analysis using a larger sample in order to clear up whether there are any differences between the evaluation at 3- and 6-months of follow-up regarding the use of probiotics. Also, the difference in the fraction of women experiencing at least one symptomatic recurrence between the two study cohorts (32.2 % vs 58.8 %) did not reach statistical significance. However, this analysis at study endpoint does not take into account the baseline differences between the two cohorts, nor captures the time-to-event effect as the Cox analysis does. The latter may be of special relevance in the study population, as it was particularly prone to recurrence (67.3 % had a history of RVVC at any time of their lives and 63.5 % reported at least one previous VVC episode within the 12 months prior to enrolment).
Variability among candidiasis and the selection of treatment are issues that have aroused considerable discussion. Recent reports suggest the possibility of two types of VVC: (a) the typical, characterized by dense vaginal discharge with occasional recurrences, and (b) the cyclic-recurrent, with hypersensitive reactions even in presence of small quantities of Candida [4]. In both cases, a strong vaginal ecosystem is desirable to fend off Candida strains and reduce the risk of VVC recurrence. Several authors suggest that maintenance therapy needs to be given frequently enough to prevent vaginal regrowth or recolonization, and transformation to a symptomatic state in cases with “host intolerance”; however, once the azole is suspended, the risk of a new episode is particularly high [8].
Witt et al. [18] found no advantages in treating RVVC with monthly 200 mg itraconazole, despite the adjuvant use of local L. gasseri lyophilisates for 6 days. Thus, in our case, the probiotic intervention was maintained over 2 months to ensure proper concentrations of lactobacilli.
Probiotics are being used in the gynaecological field as an alternative intervention for VVC and its prevention after ATB therapy, and so for bacterial vaginosis. Evidence suggests that the coaggregation of lactobacilli prevents Candida from binding to VEC [19], therefore improving the efficacy of antifungals [20, 21]. A retrospective comparative study by De Seta et al. [21] showed a better subjective resolution of typical VVC symptoms after treatment with clotrimazole 2 % vaginal cream for 3 days followed by vaginal application of L. plantarum P17630 daily for 6 days, plus a weekly application for 4 weeks, all compared with azole cream alone. However, they excluded patients with known risk factors of VVC (RVVC, prior ATB, etc.). In our study, on the contrary, no exclusion of them was considered since these women are more likely to require supplementary therapies to standard treatments.
Despite the similarities between the published evidence and our results, it should be stressed that most reports have assessed multidose azole treatment [20, 21]; hence, the effects of the L. plantarum I1001 strain may be of particular interest since use of these adjuvant vaginal tablets was associated with a significant 3-fold reduction in the HR of recurrence, during a 3-month follow-up. To our knowledge, this is the first study to report a significant effect of a probiotic in the risk of symptoms recurrence in women with high prevalence of RVVC. It is noteworthy that ATB prior to enrolment significantly increased the VVC recurrence risk, consistent with previous reports such as those from Pirotta et al. [22] and Spinillo et al. [23], who also concluded that this factor could entail even a first-episode of VVC. Their findings and those presented herein enhance the role of vaginal probiotics as a preventive strategy (complementing pharmacological treatment), and a primary intervention for reducing the risk of vaginal infections. Since ‘Prior ATB’ per se seems to significantly increase probabilities of VVC and of recurrences, L. plantarum I1001 could be of relevance as a resource for women needing to replenish their vaginal microbiota after taking ATB.
It has to be noted that, in our study, VVC relapse was considered on the basis of any self-reported sign/symptom suggesting a new episode. Despite the subjective nature of these criteria, it could probably represent a more realistic and practical criteria in daily practice, considering that the pure presence of symptoms can entail a medical consultation and/or use of over-the-counter medication. Beyond the clinical impact of the probiotic, high rates of satisfaction, tolerability and effectiveness were reported by both patients and gynaecologists.
Our study has certain limitations that must be pointed out, such as the previously commented lack of randomization and the open-label nature of the trial, thus a placebo effect cannot be ruled out. However, it is worth pointing out that the high number of women fulfilling RVVC criteria suggests they may be well aware of the symptoms of a true VVC episode. And finally, the sample size available was limited, and drop-outs during the study follow-up were not uncommon. Thus, additional studies should be conducted to evaluate the effectiveness of L. plantarum I1001, using double blind randomized designs and larger sample sizes. Also, it would be interesting to evaluate the efficacy of L. plantarum I1001 as add-on or follow-up therapy of other antifungal regimes, as well as for preventing the recurrence of bacterial vaginosis.
References
Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA (2005) Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol 43:2155–2162
Tibaldi C, Cappello N, Latino MA et al (2009) Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin Microbiol Infect 15:670–679
Peters BM, Yano J, Noverr MC, Fidel PL (2014) Candida vaginitis: when opportunism knocks, the host responds. PLoS Pathog 10:e1003965
Murina F, Graziottin A, Felice R et al (2011) The recurrent vulvovaginal candidiasis: proposal of a personalized therapeutic protocol. ISRN Obstet Gynecol 2011:806065
Marchaim D, Lemanek L, Bheemreddy S et al (2012) Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 120:1407–1414
Sobel JD (2016) Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 214:15–21
Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G et al (2015) Guideline: vulvovaginal candidosis (AWMF 015/072). S2k (excluding chronic mucocutaneous candidosis). Mycoses 58:1–15
Dovnik A, Golle A, Novak D, Arko D, Takac I (2015) Treatment of vulvovaginal candidiasis: a review of literature. Acta Dermatovenerol Alp Pannonica Adriat 24:5–7
Kovachev SM (2015) Local probiotic therapy for vaginal Candida albicans infections. Probiotics Antimicrob Proteins 7:38–44
Sobel JD, Zervos M, Reed BD, Hooton T, Soper D, Nyirjesy P et al (2003) Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob Agents Chemother 47:34–38
Food and Agriculture Organization of the United Nations and World Health Organization. 2001, posting date. Regulatory and clinical aspects of dairy probiotics. Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report. Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 30 June 2016
Hill C, Guarner F, Reid G et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Borges S, Silva J, Teixeira P (2014) The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 289:479–489
O’Hanlon DE, Moench TR, Cone RA (2013) Vaginal pH and microbiocidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8:e80074
Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC et al (2009) Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol 48:269–274
Prieto C (2016) Lactobacillus plantarum I1001 en la prevención de la candidiasis vulvovaginal recurrente. Oral communication at the Workshop SEPYP (Sociedad Española de Probióticos y Prebióticos). Seville, Spain
Atkinson MJ, Sinha A, Hass SL et al (2004) Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2:12
Witt A, Kaufmann U, Bitschnau M, Tempfer C, Ozbal A, Haytouglu E et al (2009) Monthly itraconazole versus classic homeopathy for the treatment of recurrent vulvovaginal candidiasis: a randomised trial. BJOG 116:1499–1505
Falagas ME, Betsi GI, Athanasiou S (2006) Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother 58:266–272
Ehrstrom S, Daraczy K, Rylander E et al (2010) Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect 12:691–699
De Seta F, Parazzini F, De Leo R, Banco R, Maso GP, De Santo D et al (2014) Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol 182:136–139
Pirotta M, Gunn J, Chondros P, Grover S, O'Malley P, Hurley S, Garland S (2004) Effect of lactobacillus in preventing post-antibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 329:548
Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F (1999) Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 180:14–17
Acknowledgments
The authors thank all the gynaecologists of the Instituto Palacios de Salud y Medicina de la Mujer and the Instituto de Medicina EGR Madrid who actively participated in the study and to Dr. Cindy L. Larios from the Medical Department of Clever Instruments S.L. (Barcelona) for the statistical analysis and editorial assistance for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Gynea by Kern Pharma funded the study.
Conflict of interest
J. Espadaler is a full-time employee of AB-Biotics S.A; C. Prieto is a full-time employee of Gynea by Kern Pharma.
Ethical approval
All the study materials were reviewed and approved by the Ethical Committee of the Hospital Universitario de la Princesa (Madrid, Spain).
Informed consent
Informed written consent was obtained from all participants prior to enrolment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Cox proportional-hazards regression model for recurrence of VVC at 6 months
Table S2
Average number of VVC episodes per trimester within previous 12 months compared withthose observed during follow-up
Figure S1
Symptomatic recurrence at 3 months according to use of vaginal probiotic (A) and antibiotic treatment prior to enrolment (B)
Figure S2
Event-free survival curve at 6 months after single-dose azole treatment, depending on probiotic use. Comparison of overall recurrence-free survival between patients treated with clotrimazole 500 mg single dose vs. clotrimazole + L. plantarum I1001. The difference on adjusted survival between the two cohorts achieves significance with a higher recurrence-free survival in cases with adjuvant vaginal probiotic (HR: 0.30 [95 %CI: 0.10–0.89]; P = 0.03; multivariate Cox regression model)
Figure S3
Event-free survival curve – 6 months after single-dose azole treatment, depending on history ATB prior to enrolment. Comparison of overall recurrence-free survival regarding history of antibiotic treatment prior to enrolment. The difference on adjusted survival between the two cohorts achieves significance with a markedly higher recurrence-free survival rate in cases without such risk factor (HR: 7.87 [95 %CI: 1.81-34.26]; P = 0.006, multivariate Cox regression model)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Palacios, S., Espadaler, J., Fernández-Moya, J.M. et al. Is it possible to prevent recurrent vulvovaginitis? The role of Lactobacillus plantarum I1001 (CECT7504). Eur J Clin Microbiol Infect Dis 35, 1701–1708 (2016). https://doi.org/10.1007/s10096-016-2715-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2715-8