Abstract
Background
Hereditary transthyretin (ATTRv, v for variant) amyloidosis with polyneuropathy is a rare disease caused by mutations in the transthyretin gene. In ATTRv amyloidosis, multisystem extracellular deposits of amyloid cause tissue and organ dysfunction. Patisiran is a small interfering RNA molecule drug that reduces circulating levels of mutant and wild-type TTR proteins. Prior to its regulatory approval, patisiran was available in Italy through a compassionate use programme (CUP). The aim of this study was to analyse the long-term outcomes of patients who entered into the CUP.
Methods
This was a multicentre, observational, retrospective study of patients with ATTRv amyloidosis treated with patisiran. The analysis included change from baseline to 12, 24, 36 and 48 months in familial amyloid polyneuropathy (FAP) stage, polyneuropathy disability (PND) class, neuropathy impairment score (NIS), modified body mass index (mBMI), Compound Autonomic Dysfunction Test (CADT), Karnofsky Performance Status (KPS) scale and Norfolk Quality of Life–Diabetic Neuropathy (QoL-DN) questionnaire. Safety data were also analysed.
Results
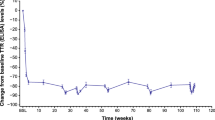
Forty patients from 11 Italian centres were enrolled: 23 in FAP 1 (6 in PND 1 and 17 in PND 2) and 17 in FAP 2 (8 in PND 3a and 9 in PND 3b) stage. In this population, the mean NIS at baseline was 71.4 (± 27.8); mBMI, 917.1 (± 207) kg/m2; KPS, 67.1 (± 14.0); Norfolk QoL-DN, 62.2 (± 25.2); and CADT, 13.2 (± 3.3). Statistical analysis showed few significant differences from baseline denoting disease stability. No new safety signals emerged.
Conclusions
Patisiran largely stabilised disease in patients with ATTRv amyloidosis.

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Luigetti M, Romano A, Di Paolantonio A, Bisogni G, Sabatelli M (2020) Diagnosis and treatment of hereditary transthyretin amyloidosis (hATTR) polyneuropathy: current perspectives on improving patient care. Ther Clin Risk Manag 16:109–123. https://doi.org/10.2147/TCRM.S219979
Koike H, Katsuno M (2020) Transthyretin amyloidosis: update on the clinical spectrum, pathogenesis, and disease-modifying therapies. Neurol Ther 9(2):317–333. https://doi.org/10.1007/s40120-020-00210-7
Coelho T, Ines M, Conceicao I, Soares M, de Carvalho M, Costa J (2018) Natural history and survival in stage 1 Val30Met transthyretin familial amyloid polyneuropathy. Neurology 91(21):e1999–e2009. https://doi.org/10.1212/WNL.0000000000006543
Adams D, Coelho T, Obici L, Merlini G, Mincheva Z, Suanprasert N, Bettencourt BR, Gollob JA, Gandhi PJ, Litchy WJ, Dyck PJ (2015) Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology 85(8):675–682. https://doi.org/10.1212/WNL.0000000000001870
Benson MD, Kincaid JC (2007) The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 36(4):411–423. https://doi.org/10.1002/mus.20821
Manganelli F, Fabrizi GM, Luigetti M, Mandich P, Mazzeo A, Pareyson D (2022) Hereditary transthyretin amyloidosis overview. Neurol Sci 43(Suppl 2):595–604. https://doi.org/10.1007/s10072-020-04889-2
Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS (2019) Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 73(22):2872–2891. https://doi.org/10.1016/j.jacc.2019.04.003
Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ (2001) Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol 159(6):1993–2000. https://doi.org/10.1016/s0002-9440(10)63050-7
Gonzalez-Duarte A (2019) Autonomic involvement in hereditary transthyretin amyloidosis (hATTR amyloidosis). Clin Auton Res 29(2):245–251. https://doi.org/10.1007/s10286-018-0514-2
Ferraro PM, D'Ambrosio V, Di Paolantonio A, Guglielmino V, Calabresi P, Sabatelli M, Luigetti M (2021) Renal involvement in hereditary transthyretin amyloidosis: an Italian single-centre experience. Brain Sci 11(8). https://doi.org/10.3390/brainsci11080980
Luigetti M, Romozzi M, Bisogni G, Cardellini D, Cavallaro T, Di Paolantonio A, Fabrizi GM, Fenu S, Gentile L, Grandis M, Marucci G, Massucco S, Mazzeo A, Pareyson D, Romano A, Russo M, Schenone A, Tagliapietra M, Tozza S, Vita G, Sabatelli M (2020) hATTR pathology: nerve biopsy results from Italian referral centers. Brain Sci 10(11):780. https://doi.org/10.3390/brainsci10110780
Luigetti M, Tortora A, Romano A, Di Paolantonio A, Guglielmino V, Bisogni G, Gasbarrini A, Calabresi P, Sabatelli M (2020) Gastrointestinal manifestations in hereditary transthyretin amyloidosis: a single-centre experience. J Gastrointestin Liver Dis 29(3):339–43. https://doi.org/10.15403/jgld-2474
Plante-Bordeneuve V, Said G (2011) Familial amyloid polyneuropathy. Lancet Neurol 10(12):1086–1097. https://doi.org/10.1016/S1474-4422(11)70246-0
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, Investigators A-AS (2018) Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379(11):1007–16. https://doi.org/10.1056/NEJMoa1805689
Cantone A, Sanguettoli F, Dal Passo B, Serenelli M, Rapezzi C (2022) The treatment of amyloidosis is being refined. Eur Heart J Suppl 24(Suppl I):I131–I138. https://doi.org/10.1093/eurheartjsupp/suac104
Padda IS, Mahtani AU, Parmar M (2023) Small Interfering RNA (siRNA) Based therapy. StatPearls [Internet]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB (2018) Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 379(1):11–21. https://doi.org/10.1056/NEJMoa1716153
Adams D, Polydefkis M, Gonzalez-Duarte A, Wixner J, Kristen AV, Schmidt HH, Berk JL, Losada Lopez IA, Dispenzieri A, Quan D, Conceicao IM, Slama MS, Gillmore JD, Kyriakides T, Ajroud-Driss S, Waddington-Cruz M, Mezei MM, Plante-Bordeneuve V, Attarian S, Mauricio E, Brannagan TH 3rd, Ueda M, Aldinc E, Wang JJ, White MT, Vest J, Berber E, Sweetser MT, Coelho T, patisiran Global OLEsg (2021) Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol 20(1):49–59. https://doi.org/10.1016/S1474-4422(20)30368-9
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB (2013) Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369(9):819–829. https://doi.org/10.1056/NEJMoa1208760
Di Stefano V, Fava A, Gentile L, Guaraldi P, Leonardi L, Poli L, Tagliapietra M, Vastola M, Fanara S, Ferrero B, Giorgi M, Perfetto F, Russo M, Russo D (2022) Italian real-life experience of patients with hereditary transthyretin amyloidosis treated with patisiran. Pharmgenomics Pers Med 15:499–514. https://doi.org/10.2147/PGPM.S359851
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, Schmidt H, Waddington-Cruz M, Campistol JM, Bettencourt BR, Vaishnaw A, Gollob J, Adams D (2015) Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis 10:109. https://doi.org/10.1186/s13023-015-0326-6
Luigetti M, Wixner J, Ueda M, Marquez W Jr, Dalia S, Arum S, Hale C, Jay P, Capocelli K, Adams D (2023) Patisiran global open-label extension study at 36 months: effect of long-term treatment on mortality and ambulatory function in patients with hATTR amyloidosis with polyneuropathy. J Peripher Nerv Syst 28(Suppl 2):S35. https://doi.org/10.1111/jns.12550
Gentile L, Russo M, Luigetti M, Bisogni G, Di Paolantonio A, Romano A, Guglielmino V, Arimatea I, Sabatelli M, Toscano A, Vita G, Mazzeo A (2021) Patisiran in hATTR amyloidosis: six-month latency period before efficacy. Brain Sci 11(4):515. https://doi.org/10.3390/brainsci11040515
Coutinho PM, Martins da Silva A, Lopes JL, Barbarosa AR (1980) Forty years of experience with type I amyloid neuropathy. Review of 483 cases. In: Glenner GG, Costa PP, de Freitas AF (eds) Amyloid amyloidosis. Excerpta Medica, Amsterdam. https://www.spmi.pt/wp-content/uploads/2019/10/9.-Amyloid-and-Amyloidosis-1979.pdf
Yamamoto S, Wilczek HE, Nowak G, Larsson M, Oksanen A, Iwata T, Gjertsen H, Soderdahl G, Wikstrom L, Ando Y, Suhr OB, Ericzon BG (2007) Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 7(11):2597–2604. https://doi.org/10.1111/j.1600-6143.2007.01969.x
Dyck PJ, Boes CJ, Mulder D, Millikan C, Windebank AJ, Dyck PJ, Espinosa R (2005) History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J Peripher Nerv Syst 10(2):158–73. https://doi.org/10.1111/j.1085-9489.2005.0010206.x
Denier C, Ducot B, Husson H, Lozeron P, Adams D, Meyer L, Said G, Plante-Bordeneuve V (2007) A brief compound test for assessment of autonomic and sensory-motor dysfunction in familial amyloid polyneuropathy. J Neurol 254(12):1684–1688. https://doi.org/10.1007/s00415-007-0617-5
Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM (ed) Evaluation of chemotherapeutic agents. Columbia University Press, New York, p 196
Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford-Molvik SL, Vinik AI (2005) The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 7(3):497–508. https://doi.org/10.1089/dia.2005.7.497
ClinTrials.gov. The study of an investigational drug, patisiran (ALN-TTR02), for the treatment of transthyretin (TTR)-mediated amyloidosis in participants who have already been treated with ALN-TTR02 (patisiran) [Internet]. ClinicalTrials.gov. 2023. Available from: https://clinicaltrials.gov/study/NCT02510261?tab=results#outcome-measures. Accessed 26 Feb 2024
de Frutos F, Ochoa JP, Gomez-Gonzalez C, Reyes-Leiva D, Arostegui JI, Casasnovas C, Barriales-Villa R, Sevilla T, Gonzalez-Lopez E, Ramil E, Galan L, Gonzalez-Costello J, Garcia-Alvarez A, Rojas-Garcia R, Espinosa MA, Garcia-Pavia P (2023) Phenotype and clinical outcomes of Glu89Lys hereditary transthyretin amyloidosis: a new endemic variant in Spain. Amyloid 30(2):199–207. https://doi.org/10.1080/13506129.2022.2142110
Labeyrie C, Merkel M, Sethi S, Popadic L, Yang H, Lin H, Adams D (2022) Effectiveness of patisiran following switch from tafamidis for the treatment of heredirary transthyretin-mediatied (hATTR) amyloidosis with polyneuropathy. XVIII International Symposium on Amyloidosis, 4th–8th September 2022, Heidelberg, Germany
Quan D, Obici L, Berk JL, Ando Y, Aldinc E, White MT, Adams D (2023) Impact of baseline polyneuropathy severity on patisiran treatment outcomes in the APOLLO trial. Amyloid 30(1):49–58. https://doi.org/10.1080/13506129.2022.2118043
De Bleecker JL, Claeys KG, Delstanche S, Van Parys V, Baets J, Tilleux S, Remiche G (2023) A retrospective survey of patients with hereditary transthyretin-mediated (hATTR) amyloidosis treated with patisiran in real-world clinical practice in Belgium. Acta Neurol Belg 123(3):1029–1037. https://doi.org/10.1007/s13760-023-02188-z
Schmidt HH, Wixner J, Plante-Bordeneuve V, Munoz-Beamud F, Llado L, Gillmore JD, Mazzeo A, Li X, Arum S, Jay PY, Adams D, Patisiran Post LTSG (2022) Patisiran treatment in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy after liver transplantation. Am J Transplant 22(6):1646–57. https://doi.org/10.1111/ajt.17009
Yarlas A, Gertz MA, Dasgupta NR, Obici L, Pollock M, Ackermann EJ, Lovley A, Kessler AS, Patel PA, White MK, Guthrie SD (2019) Burden of hereditary transthyretin amyloidosis on quality of life. Muscle Nerve 60(2):169–175. https://doi.org/10.1002/mus.26515
Brannagan TH 3rd, Auer-Grumbach M, Berk JL, Briani C, Bril V, Coelho T, Damy T, Dispenzieri A, Drachman BM, Fine N, Gaggin HK, Gertz M, Gillmore JD, Gonzalez E, Hanna M, Hurwitz DR, Khella SL, Maurer MS, Nativi-Nicolau J, Olugemo K, Quintana LF, Rosen AM, Schmidt HH, Shehata J, Waddington-Cruz M, Whelan C, Ruberg FL (2021) ATTR amyloidosis during the COVID-19 pandemic: insights from a global medical roundtable. Orphanet J Rare Dis 16(1):204. https://doi.org/10.1186/s13023-021-01834-0
Gonzalez-Duarte A, Berk JL, Quan D, Mauermann ML, Schmidt HH, Polydefkis M, Waddington-Cruz M, Ueda M, Conceicao IM, Kristen AV, Coelho T, Cauquil CA, Tard C, Merkel M, Aldinc E, Chen J, Sweetser MT, Wang JJ, Adams D (2020) Analysis of autonomic outcomes in APOLLO, a phase III trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. J Neurol 267(3):703–712. https://doi.org/10.1007/s00415-019-09602-8
EMA. ONPATTRO, INN-patisiran. Summary of Product Characteristics (2018) [Available from: https://www.ema.europa.eu/en/documents/product-information/onpattro-epar-product-information_en.pdf.] Accessed 20 Jun 2023
Acknowledgements
The paper was written by Alicja M. Gruszka, an independent medical writer on behalf of Springer Healthcare. This writing assistance was sponsored by Alnylam.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Luca Gentile, Anna Mazzeo, Fiore Manganelli, Laura Obici, Marco Luigetti. Methodology: Luca Gentile, Anna Mazzeo, Fiore Manganelli, Laura Obici, Marco Luigetti. Formal analysis and investigation: Luca Gentile, Anna Mazzeo, Fiore Manganelli, Laura Obici, Marco Luigetti. Writing—original draft preparation: Luca Gentile, Anna Mazzeo, Fiore Manganelli, Laura Obici, Marco Luigetti. Writing—review and editing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr Luigetti received financial grants (honoraria and speaking) from Ackea, Alnylam, Sobi, and Pfizer, and travel grants from Ackea, Alnylam, Sobi, Pfizer, Kedrion and Grifols.
Dr. Gentile received financial grants (honoraria and speaking) from Akcea, Alnylam, and Pfizer, and travel grants from Alnylam and CSL Behring.
Dr. Mazzeo received speaker fees and consulting honoraria from Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer Inc.
Dr. Manganelli has no conflict of interest to declare.
Dr Obici reports speaker and consulting honoraria from Pfizer, Alnylam, SOBI, Novo Nordisk, Astra Zeneca and BridgeBio.
Dr. Briani received speaker and consulting honoraria from Alnylam, Ionis, and Pfizer, and travel grants from Kedrion, Alnylam and CSL Behring.
Dr. Casagrande has no conflict of interest to declare.
Dr. De Luca received financial grants (speaking) from Pfizer, and travel grants to attend scientific meetings from Pfizer and SOBI.
Dr. Fabrizi received financial grants (honoraria and speaking) from Akcea, Alnylam and travel grants from Akcea, Pfizer and Kedrion.
Dr. Gagliardi received financial grants (consulting and speaking) from Pfizer and Alnylam.
Dr. Gemelli received financial grants (speaking) from Italfarmaco, and travel grants from Biogen for participation in national meetings.
Dr. Forcina has no conflict of interest to declare.
Dr. Grandis acknowledges donations from Sanofi Genzyme to support research activities of her Research Unit. Financial support from Pfizer, Alnylam and Sanofi Genzyme for participation in National and International Meetings. Participation in Advisory Board of Pfizer and Argenx. Speaker honorarium from Sanofi Genzyme and Alexion.
Dr. Guglielmino has no conflict of interest to declare.
Dr. Iabichella received travel grants to attend scientific meetings from Alnylam and SOBI.
Dr. Leonardi received financial grants (honoraria and speaking) from Alnylam, and travel grants to attend scientific meetings from Akcea, SOBI, Alnylam.
Dr. Lozza has no conflict of interest to declare.
Dr. Mussinelli received financial grants (speaking) from Pfizer and SOBI, and travel grants to attend scientific meetings from Alnylam.
Dr. My received financial grants (honoraria and speaking) from Alnylam, and travel grants to attend scientific meetings from Alnylam.
Dr. Occhipinti has no conflict of interest to declare.
Dr. Fenu acknowledges financial support from Alnylam and Pfizer for participation in national and international meetings.
Dr. Russo has no conflict of interest to declare.
Dr. Romano received financial grants (honoraria and speaking) from Akcea, and travel grants to attend scientific meetings from Akcea, Alnylam, Pfizer, and Csl Behring.
Dr. Salvalaggio received travel grant to attend scientific meeting from Alnylam.
Dr. Stefano Tozza received personal fees for scientific events from Alnylam Pharmaceuticals, Amicus Therapeutics and Takeda Pharmaceutical Co, travel grants to attend scientific meetings from Akcea Therapeutics.
Ethical approval
The study protocol was approved by Ethics Committee of Unit of Neurology and Neuromuscular Diseases, AOU G. Martino, Messina, i.e., the coordinating centre (protocol number: 109-21) and subsequently by the Ethics Committees of the participating centres.
Informed consent
Participants provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gentile, L., Mazzeo, A., Briani, C. et al. Long-term treatment of hereditary transthyretin amyloidosis with patisiran: multicentre, real-world experience in Italy. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07494-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07494-9