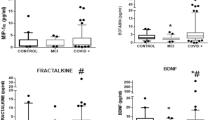
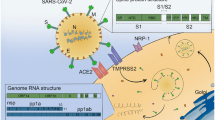
Abstract
Background
COVID-19 is a disease known for its neurological involvement. SARS-CoV-2 infection triggers neuroinflammation, which could significantly contribute to the development of long-term neurological symptoms and structural alterations in the gray matter. However, the existence of a consistent pattern of cerebral atrophy remains uncertain.
Objective
Our study aimed to identify patterns of brain involvement in recovered COVID-19 patients and explore potential relationships with clinical variables during hospitalization.
Methodology
In this study, we included 39 recovered patients and 39 controls from a pre-pandemic database to ensure their non-exposure to the virus. We obtained clinical data of the patients during hospitalization, and 3 months later; in addition we obtained T1-weighted magnetic resonance images and performed standard screening cognitive tests.
Results
We identified two groups of recovered patients based on a cluster analysis of the significant cortical thickness differences between patients and controls. Group 1 displayed significant cortical thickness differences in specific cerebral regions, while Group 2 exhibited significant differences in the cerebellum, though neither group showed cognitive deterioration at the group level. Notably, Group 1 showed a tendency of higher D-dimer values during hospitalization compared to Group 2, prior to p-value correction.
Conclusion
This data-driven division into two groups based on the brain structural differences, and the possible link to D-dimer values may provide insights into the underlying mechanisms of SARS-COV-2 neurological disruption and its impact on the brain during and after recovery from the disease.


Similar content being viewed by others
Data availability
All the data supporting our findings are contained within the manuscript. De-identified data to replicate our results will be available to qualified researchers upon written request to the corresponding author.
References
Hu B, Guo H, Zhou P, Shi Z-L (2021) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154. https://doi.org/10.1038/s41579-020-00459-7
Elrobaa IH, New KJ (2021) COVID-19: pulmonary and extra pulmonary manifestations. Front Public Health 9:711616. https://doi.org/10.3389/fpubh.2021.711616
Shen Q, Li J, Zhang Z, Guo S, Wang Q, An X, Chang H (2022) COVID-19: systemic pathology and its implications for therapy. Int J Biol Sci 18:386–408. https://doi.org/10.7150/ijbs.65911
Al-Sarraj S, Troakes C, Hanley B, Osborn M, Richardson MP, Hotopf M, Bullmore E, Everall IP (2021) Invited review: the spectrum of neuropathology in COVID-19. Neuropathol Appl Neurobiol 47:3–16. https://doi.org/10.1111/nan.12667
Di Carlo DT, Montemurro N, Petrella G, Siciliano G, Ceravolo R, Perrini P (2021) Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: a systematic review of current literature. J Neurol 268:1561–1569. https://doi.org/10.1007/s00415-020-09978-y
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG (2020) Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 369:m1985. https://doi.org/10.1136/bmj.m1985
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77:683. https://doi.org/10.1001/jamaneurol.2020.1127
Ferrucci R, Dini M, Groppo E, Rosci C, Reitano MR, Bai F, Poletti B, Brugnera A, Silani V, D’Arminio Monforte A, Priori A (2021) Long-lasting cognitive abnormalities after COVID-19. Brain Sci 11:235. https://doi.org/10.3390/brainsci11020235
Mendelson M, Nel J, Blumberg L, Madhi SA, Dryden M, Stevens W, Venter FWD (2021) Long-COVID: an evolving problem with an extensive impact. S Afr Med J 111:10–12. https://doi.org/10.7196/SAMJ.2021.v111i1.15433
Mishra SS, Hafiz R, Misra R, Gandhi TK, Prasad A, Mahajan V, Biswal BB (2022) Brain Alterations in COVID recovered revealed by susceptibility-weighted magnetic resonance imaging.https://doi.org/10.1101/2022.11.21.22282600
Kumar M, Thakur AK (2020) Neurological manifestations and comorbidity associated with COVID-19: an overview. Neurol Sci 41:3409–3418. https://doi.org/10.1007/s10072-020-04823-6
Woo MS, Shafiq M, Fitzek A, Dottermusch M, Altmeppen H, Mohammadi B, Mayer C, Bal LC, Raich L, Matschke J, Krasemann S, Pfefferle S, Brehm TT, Lütgehetmann M, Schädler J, Addo MM, Schulze zurWiesch J, Ondruschka B, Friese MA, Glatzel M, (2023) Vagus nerve inflammation contributes to dysautonomia in COVID-19. Acta Neuropathol (Berl) 146:387–394. https://doi.org/10.1007/s00401-023-02612-x
Cacciola R, GentiliniCacciola E, Vecchio V, Cacciola E (2022) Cellular and molecular mechanisms in COVID-19 coagulopathy: role of inflammation and endotheliopathy. J Thromb Thrombolysis 53:282–290. https://doi.org/10.1007/s11239-021-02583-4
Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC). Cardiovasc Diabetol 21:148. https://doi.org/10.1186/s12933-022-01579-5
Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O’Sullivan JM, Martin-Loeches I, Nadarajan P, Bannan C, Mallon PW, Curley GF, Preston RJS, Rehill AM, McGonagle D, Ni Cheallaigh C, Baker RI, Renné T, Ward SE, O’Donnell JS, Investigators the IC-19 VS (iCVS) (2021) Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 19:2546–2553. https://doi.org/10.1111/jth.15490
Wan D, Du T, Hong W, Chen L, Que H, Lu S, Peng X (2021) Neurological complications and infection mechanism of SARS-CoV-2. Signal Transduct Target Ther 6:406. https://doi.org/10.1038/s41392-021-00818-7
Vanderheiden A, Klein RS (2022) Neuroinflammation and COVID-19. Curr Opin Neurobiol 76:102608. https://doi.org/10.1016/j.conb.2022.102608
Besteher B, Machnik M, Troll M, Toepffer A, Zerekidze A, Rocktäschel T, Heller C, Kikinis Z, Brodoehl S, Finke K, Reuken PA, Opel N, Stallmach A, Gaser C, Walter M (2022) Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res 317:114836. https://doi.org/10.1016/j.psychres.2022.114836
Tremblay M-E, Madore C, Bordeleau M, Tian L, Verkhratsky A (2020) Neuropathobiology of COVID-19: the role for glia. Front Cell Neurosci 14:1–15. https://doi.org/10.3389/fncel.2020.592214
Tsagkaris C, Bilal M, Aktar I, Aboufandi Y, Tas A, Aborode AT, Suvvari TK, Ahmad S, Shkodina A, Phadke R, Emhamed MS, Baig AA, Alexiou A, Ashraf GMd, Kamal MA (2022) Cytokine storm and neuropathological alterations in patients with neurological manifestations of COVID-19. Curr Alzheimer Res 19:641–657. https://doi.org/10.2174/1567205019666220908084559
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian D-S (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71:762–768. https://doi.org/10.1093/cid/ciaa248
Hong L-Z, Shou Z-X, Zheng D-M, Jin X (2021) The most important biomarker associated with coagulation and inflammation among COVID-19 patients. Mol Cell Biochem 476:2877–2885. https://doi.org/10.1007/s11010-021-04122-4
Lai Y-J, Liu S-H, Manachevakul S, Lee T-A, Kuo C-T, Bello D (2023) Biomarkers in long COVID-19: a systematic review. Front Med 10:1–10. https://doi.org/10.3389/fmed.2023.1085988
Coomes EA, Haghbayan H (2020) Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol 30:e2141. https://doi.org/10.1002/rmv.2141
Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, Martin KA, Chun HJ, Hwa J (2021) Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol 18:194–209. https://doi.org/10.1038/s41569-020-00469-1
Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A (2021) Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 21:319–329. https://doi.org/10.1038/s41577-021-00536-9
Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, Bergin C, O’Farrelly C, Conlon N, Bourke NM, Ward SE, Byrne M, Ryan K, O’Connell N, O’Sullivan JM, Ni Cheallaigh C, O’Donnell JS (2021) Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost 19:1064–1070. https://doi.org/10.1111/jth.15267
Lu Y, Li X, Geng D, Mei N, Wu P-Y, Huang C-C, Jia T, Zhao Y, Wang D, Xiao A, Yin B (2020) Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. eClinicalMedicine 25. https://doi.org/10.1016/j.eclinm.2020.100484
Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604:697–707. https://doi.org/10.1038/s41586-022-04569-5
Manca R, De Marco M, Ince PG, Venneri A (2021) Heterogeneity in regional damage detected by neuroimaging and neuropathological studies in older adults with COVID-19: a cognitive-neuroscience systematic review to inform the long-term impact of the virus on neurocognitive trajectories. Front Aging Neurosci 13:1–29. https://doi.org/10.3389/fnagi.2021.646908
Díez-Cirarda M, Yus M, Gómez-Ruiz N, Polidura C, Gil-Martínez L, Delgado-Alonso C, Jorquera M, Gómez-Pinedo U, Matias-Guiu J, Arrazola J, Matias-Guiu JA (2023) Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 146:2142–2152. https://doi.org/10.1093/brain/awac384
Du Y, Zhao W, Huang S, Huang Y, Chen Y, Zhang H, Guo H, Liu J (2023) Two-year follow-up of brain structural changes in patients who recovered from COVID-19: a prospective study. Psychiatry Res 319:114969. https://doi.org/10.1016/j.psychres.2022.114969
Manjón JV, Coupé P (2016) volBrain: an online MRI brain volumetry system. Front Neuroinformatics 10:1–14. https://doi.org/10.3389/fninf.2016.00030
Manjón JV, Romero JE, Vivo-Hernando R, Rubio G, Aparici F, de la Iglesia-Vaya M, Coupé P (2022) vol2Brain: a new online pipeline for whole brain MRI analysis. Front Neuroinformatics 16:1–11. https://doi.org/10.3389/fninf.2022.862805
Romero JE, Coupé P, Giraud R, Ta V-T, Fonov V, Park MTM, Chakravarty MM, Voineskos AN, Manjón JV (2017) CERES: a new cerebellum lobule segmentation method. Neuroimage 147:916–924. https://doi.org/10.1016/j.neuroimage.2016.11.003
Sanchis-Segura C, Ibañez-Gual MV, Aguirre N, Cruz-Gómez ÁJ, Forn C (2020) Effects of different intracranial volume correction methods on univariate sex differences in grey matter volume and multivariate sex prediction. Sci Rep 10:12953. https://doi.org/10.1038/s41598-020-69361-9
Guàrdia-Olmos J, Peró-Cebollero M, Rivera D, Arango-Lasprilla JC (2015) Methodology for the development of normative data for ten Spanish-language neuropsychological tests in eleven Latin American countries. NeuroRehabilitation 37:493–499. https://doi.org/10.3233/NRE-151277
Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG (2011) The cerebellar cognitive profile. Brain 134:3672–3686. https://doi.org/10.1093/brain/awr266
García-Grimshaw M, Chirino-Pérez A, Flores-Silva FD, Valdés-Ferrer SI, Vargas-Martínez M de los Á, Jiménez-Ávila AI, Chávez-Martínez OA, Ramos-Galicia EM, Marché-Fernández OA, Ramírez-Carrillo MF, Grajeda-González SL, Ramírez-Jiménez ME, Chávez-Manzanera EA, Tusié-Luna MT, Ochoa-Guzmán A, Cantú-Brito C, Fernandez-Ruiz J, Chiquete E (2022) Critical role of acute hypoxemia on the cognitive impairment after severe COVID-19 pneumonia: a multivariate causality model analysis. Neurol Sci 43:2217–2229. https://doi.org/10.1007/s10072-021-05798-8
Yamga E, Mullie L, Durand M, Cadrin-Chenevert A, Tang A, Montagnon E, Chartrand-Lefebvre C, Chassé M (2023) Interpretable clinical phenotypes among patients hospitalized with COVID-19 using cluster analysis. Front Digit Health 5:1–13. https://doi.org/10.3389/fdgth.2023.1142822
Bougakov D, Podell K, Goldberg E (2021) Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol 58:564–575. https://doi.org/10.1007/s12035-020-02152-5
Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA (2021) Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care 34:1062–1071. https://doi.org/10.1007/s12028-020-01049-4
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel H-H, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL (2021) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. https://doi.org/10.1038/s41593-020-00758-5
Zhu Y, Chen X, Liu X (2022) NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and Beyond. Front Immunol 13:1–11. https://doi.org/10.3389/fimmu.2022.838011
Bivona G, Agnello L, Ciaccio M (2021) Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med 41:540–548. https://doi.org/10.3343/alm.2021.41.6.540
Eljilany I, Elzouki A-N (2020) D-Dimer, Fibrinogen, and IL-6 in COVID-19 patients with suspected venous thromboembolism: a narrative review. Vasc Health Risk Manag 16:455–462. https://doi.org/10.2147/VHRM.S280962
Johnson MH, Christman CW (1995) Posterior circulation infarction: anatomy, pathophysiology, and clinical correlation. Semin Ultrasound CT MRI 16:237–252. https://doi.org/10.1016/0887-2171(95)90020-9
Lentsch AB, Ward PA (2000) Regulation of inflammatory vascular damage. J Pathol 190:343–348. https://doi.org/10.1002/(SICI)1096-9896(200002)190:3%3c343::AID-PATH522%3e3.0.CO;2-M
Parvez MSA, Ohtsuki G (2022) Acute cerebellar inflammation and related ataxia: mechanisms and pathophysiology. Brain Sci 12:367. https://doi.org/10.3390/brainsci12030367
Sawaishi Y, Takada G (2002) Acute cerebellitis. The Cerebellum 1:223–228. https://doi.org/10.1080/14734220260418457
Kipp M, Norkute A, Johann S, Lorenz L, Braun A, Hieble A, Gingele S, Pott F, Richter J, Beyer C (2008) Brain-region-specific astroglial responses in vitro after LPS exposure. J Mol Neurosci 35:235–243. https://doi.org/10.1007/s12031-008-9057-7
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediators Inflamm 2013:1–19. https://doi.org/10.1155/2013/342931
Escartin C, Guillemaud O, Carrillo-de Sauvage M-A (2019) Questions and (some) answers on reactive astrocytes. Glia 67:2221–2247. https://doi.org/10.1002/glia.23687
Kang K, Han J, Lee S-W, Jeong SY, Lim Y-H, Lee J-M, Yoon U (2020) Abnormal cortical thickening and thinning in idiopathic normal-pressure hydrocephalus. Sci Rep 10:21213. https://doi.org/10.1038/s41598-020-78067-x
Zimmermann N, Goulart Corrêa D, Tukamoto G, Netto T, Batista Pereira D, Paz Fonseca R, Gasparetto EL (2017) Brain morphology and cortical thickness variations in systemic lupus erythematosus patients: differences among neurological, psychiatric, and nonneuropsychiatric manifestations. J Magn Reson Imaging 46:150–158. https://doi.org/10.1002/jmri.25538
Batzu L, Westman E, Pereira JB (2020) Cerebrospinal fluid progranulin is associated with increased cortical thickness in early stages of Alzheimer’s disease. Neurobiol Aging 88:61–70. https://doi.org/10.1016/j.neurobiolaging.2019.12.012
Alvarez JA, Emory E (2006) Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16:17–42. https://doi.org/10.1007/s11065-006-9002-x
Herlin B, Navarro V, Dupont S (2021) The temporal pole: from anatomy to function—a literature appraisal. J Chem Neuroanat 113:101925. https://doi.org/10.1016/j.jchemneu.2021.101925
Baillieux H, Smet HJD, Paquier PF, De Deyn PP, Mariën P (2008) Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg 110:763–773. https://doi.org/10.1016/j.clineuro.2008.05.013
Timmann D, Daum I (2007) Cerebellar contributions to cognitive functions: a progress report after two decades of research. The Cerebellum 6:159–162. https://doi.org/10.1080/14734220701496448
Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B (2022) Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA 327:583–584. https://doi.org/10.1001/jama.2021.24868
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, Wang M, Mao Q (2022) Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med 54:516–523. https://doi.org/10.1080/07853890.2022.2034936
Thakur M, Datusalia AK, Kumar A (2022) Use of steroids in COVID-19 patients: a meta-analysis. Eur J Pharmacol 914:174579. https://doi.org/10.1016/j.ejphar.2021.174579
Funding
This work received funding from CONAHCYT–Mexico grant no. A1-S-10669 and PAPIIT-UNAM grant no. IN214122 given to Juan Fernandez-Ruiz and CONAHCYT–Mexico Ph.D. fellowship no. 789431 given to Angel Omar Romero Molina (CVU: 782944).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all the authors. The first draft of the manuscript was written by Angel Omar Romero-Molina, and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Declarations
Ethical approval and consent to participate
This project was approved by the research and ethical committee of the Instituto Nacional de Enfermedades Respiratorias under approval number C48-20 and the Instituto Nacional de Neurologia y Neurocirugia under approval number 06–21. Signed informed consent was obtained from all participants and the procedures about human research were according to the Helsinki declaration.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romero-Molina, A.O., Ramirez-Garcia, G., Chirino-Perez, A. et al. SARS-CoV-2’s brain impact: revealing cortical and cerebellar differences via cluster analysis in COVID-19 recovered patients. Neurol Sci 45, 837–848 (2024). https://doi.org/10.1007/s10072-023-07266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07266-x