Abstract
Objectives
The aim of this review is to provide an overview on prevalence and clinical tools for the diagnosis of apathy, as well as on neurophysiological and neuroimaging findings obtained from studies in patients with apathy in different forms of dementia, including Alzheimer’s disease (AD), vascular (VaD) and mixed dementia, frontotemporal dementia (FTD), and Parkinson’s disease dementia (PDD).
Methods
Randomized controlled trials, non-randomized controlled trials, controlled before–after studies, and interrupted time series from four databases (WebOfScience, Scopus, Pubmed, and PsycINFO) addressing apathy in adults or older people aged over 65 years of age affected by dementia were included.
Results
The prevalence of apathy was 26–82% for AD, 28.6–91.7 for VaD, 29–97.5% in PDD, and 54.8–88.0 in FTD. The assessment of apathy was not consistent in the reviewed studies. Methylphenidate was the most successful pharmacological treatment for apathy. Neurobiological studies highlighted the relationship between both structural and functional brain areas and the presence or severity of apathy.
Conclusion
Apathy is a very common disorder in all types of dementia, although it is often underdiagnosed and undertreated. Further studies are needed to investigate its diagnosis and management. A consensus on the different evaluation scales should be achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apathy is defined as a decrease in “goal-directed” behavior and impaired motivation not determined by a diminished level of consciousness, cognitive impairment, emotional distress, and depression [1, 2]. Apathy can be a symptom of neurological or psychiatric conditions, although it was recently detected also in older adults with healthy cognitive functions [3], and can display three different phenotypes. “Emotional-affective” apathy is defined as the failure to create the required relationship between emotional-affective impulses and current or future conduct; “cognitive” apathy refers to difficulties in elaborating the plan of actions required for the ongoing or future behavior; “auto-activation” apathy refers to the inability to self-activate thoughts or self-initiate actions, as opposed to a relatively spared ability to generate externally driven behavior [4]. Apathy can be consistently found in several neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease dementia (PDD), frontotemporal dementia (FTD), or vascular dementia (VaD) [5]. Its prevalence increases during disease progression [6], and it is linked to faster cognitive deterioration and increased risk of institutionalization [7]. Overall, existing evidence shows that apathy strongly impacts on the quality of life of both patients and caregivers [8]. Nevertheless, data on apathy in different forms of dementia are sparse [9,10,11]: there is no agreement on a recommended tool for apathy assessment [12, 13], it is rarely investigated as a primary outcome in literature [14,15,16], its neurobiological correlates are unclear, and evidence on possible treatments is limited [17]. For the above reasons, apathy in dementia is often misdiagnosed and undertreated [18, 19].
The aim of this paper is to provide an overview on prevalence, assessment tools, neurophysiological and neuroimaging correlates, and therapeutic options for apathy in different forms of dementia including AD, PDD, FTD, and VaD. We will identify gaps in the literature and future perspectives and provide information for a proper management of apathy, to improve the quality of life of both patients and caregivers.
Methods
The following databases were used for this narrative review: WebOfScience, Scopus, Pubmed, and PsycINFO. The script used for the search is (apathy) AND (dementia). General keywords focusing on the role of apathy in dementia were included.
Inclusion and exclusion criteria
We included randomized (RCT) and non-randomized clinical trials, controlled before–after studies, and interrupted time series addressing diagnosis, assessment, or treatment of apathy in dementia. Meta-analysis, systematic reviews, and animal models were excluded. Abstracts and full texts from the original search were reviewed individually by different authors. Disagreement at the screening stage was resolved by including a third author at the full-text stage, if required. Studies on subjects with non-neurodegenerative diseases, head trauma, stroke, brain tumors, and psychiatric diseases, as well as those not addressing apathy were excluded. Inclusion criteria entailed studies on adults or older adults (aged over 65 years old) and the presence of cognitive impairment. The research included only articles in English language published after 2017.
Data extraction
Data from individual studies were extracted in accordance with the PICOS approach [20]. The following information was extracted from each study: (1) author/s and year of publication, type of study, (2) characteristics of the participants, (3) cognitive and/or psychological domains investigated, (4) apathy evaluation, (5) type of treatment or intervention, and (6) primary outcome of the study. Continuous variables are expressed as value ± standard deviation, while categorical variables as absolute number and/or percentage.
Results
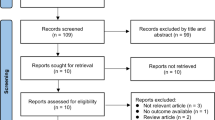
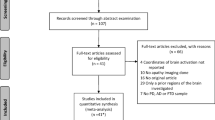
The research produced 3570 articles. A total of 836 records were found in Web of Science, 1266 records were identified on Scopus, 533 records were identified through Pubmed, and 935 records were identified on PsycInfo. Identified records were entered into the Mendeley software for their management and elimination of duplicates. A total of 2223 records were screened according to title and abstract. After 1821 records were excluded, 402 articles were evaluated for full-text. Therefore, 102 articles were selected according to eligibility criteria (records excluded 300). Seventy-five articles were included in the qualitative analysis (Fig. 1). We described the main results, according to each major clinical disease. For each clinical condition, after a preliminary description, we reported prevalence, available treatments, tools used for apathy assessment, and results from neuroimaging and functional studies.
Alzheimer’s disease
AD is a neurodegenerative disease characterized by the accumulation of neuropathological abnormalities such as β-amyloid plaques and neurofibrillary tangles. The prodromal phase of this disease involves neural loss, typically affecting the hippocampus, leading to a progressive atrophy in large-scale of networks widely undermining cognitive and neuropsychiatric functions. AD negatively affects activities of daily living with loss of motivation and interest in several aspects of life. A summary of studies addressing apathy in AD included in the review is shown in Table 1.
AD—prevalence
Thirty-nine articles met the inclusion criteria for apathy in AD. Five of them investigated AD together with bvFTD [12, 21,22,23,24], primary progressive aphasia (PPA) [23], and subcortical ischemic vascular disease (SIVD) [13]. The prevalence of apathy varied from 26 to 82%, and it increases, along with its severity, with the progression of cognitive and functional decline and brain atrophy.
AD—apathy assessment
Seven apathy-specific scales were used: Apathy Evaluation Scale (AES) [13, 23, 25,26,27,28,29,30,31], Dementia Apathy Interview and Rating (DAIR) [32, 33], Dimensional Apathy Scale (DAS) [22, 34, 35], Lille Apathy Rating Scale (LARS) [12], Apathy Rating Scale (ARS) [36], Apathy in Dementia-Nursing Home Version Scale (AES-NH) [37]. Seven apathy subscales were included in more global questionnaires, such as Neuropsychiatric Inventory (NPI) [12, 21, 24, 32, 33, 38,39,40,41,42,43,44,45,46,47,48,49,50] or Frontal Behavioral Inventory (FBI-a) [12]. The most utilized tools were AES and NPI. Using AES, the highest score was reported in a sample of 32 patients (mean age 84.5 ± 9.5 years old) and was 56.5 ± 13.0 points [31], while the lowest score of 34.4 ± 13.40 points was found in a sample of 34 patients (with a lower mean age of 77.8 ± 6.51 years old) [13]. The highest NPI score was 7.8 ± 2.4, and it was observed in a sample of 200 patients aged 76 ± 5 years old [45], and the lowest NPI score was 0.6 ± 0.8, and it was reported in a sample of 95 subjects aged 75.6 ± 7.4 years [39]. In a large sample of 1925 patients suffering from AD, apathy was diagnosed in more than two third of the sample [47].
AD—apathy treatment
Nine studies addressed different therapeutic options, and methylphenidate was the most investigated treatment.
Methylphenidate
Efficacy of methylphenidate was investigated by two RCTs. Padala and colleagues demonstrated an increase on the clinician/researcher version of the AES after 12 weeks of treatment with 10 mg/day of methylphenidate. A greater improvement in apathy in multiple domains (behavioral, cognitive, emotional, and motivation), global cognition, functional status, caregiver burden, and depression started after 4 weeks [51]. Mintzer et al. in a 6-month RCT demonstrated significant beneficial effects of 20 mg/day methylphenidate on apathy. The largest decrease in the NPI apathy score was observed during the first 100 days. Conversely, no further improvements in cognitive measures and quality of life was reported after 6 months [45].
Sertaline
An RCT investigated the efficacy of sertraline, escitalopram, and nicergoline to treat apathy and depression in AD. Regarding apathy, the authors observed a significant improvement with sertraline only [52].
Ninjin’yoeito (NYT, TJ-108)
An open-label pilot study reported significant positive effects on apathy after using Ninjin’yoeito (NYT, TJ-108) in 20 AD subjects. NYT, TJ-108 is a traditional Japanese multicomponent herbal medicine. The authors reported an increase on NPI apathy and anorexia subscales and global cognitive functions [38].
BrainUp-10® (BU-10)
An RCT by Guzman-Martinez and colleagues investigated the effect of BrainUp-10® (BU-10) in 74 AD subjects. After 24 weeks of treatment, the authors obtained a statistically significant improvement of apathy. In contrast, no differences on cognitive functions were observed [53].
Oil diluted cannabis extract (Bedrocan)
Palmieri and Vadalà demonstrated benefits on apathy-subdomain score of NPI and cognitive status assessed by mini-mental state examination (MMSE) (in 45% of the sample), as well as on the caregiver’s quality of life, 3 months after the administration of Bedrocan [49].
Stimulation techniques
Two studies investigated the effect of repetitive transcranial magnetic stimulation (rTMS), showing significant effects on apathy [30, 40]. Nguyen and colleagues examined the efficacy of 5 weeks of rTMS combined with cognitive training in a sample of 10 AD patients. rTMS was delivered over 6 brain areas, namely, right and left dorsolateral pre-frontal cortex (DLPFC), right and left posterior parietal cortex associative areas, and Broca and Wernicke language areas, and it was combined with cognitive tasks stimulating the three different cortical regions (spatial attention tasks for the parietal cortex, naming of actions and objects, word recall and spatial memory tasks for the prefrontal cortex, and syntax and grammar tasks for language areas). For each region, 40 s of cognitive training were performed between each train of 10 Hz-rTMS stimulation. A series of 20 trains per session was administered, and the patients received 3 sessions. At the end of the treatment, a significant improvement of apathy, disability, and cognitive functions was observed [40]. A study on 20 AD patients by Padala and colleagues reported significantly greater improvement of apathy and general cognitive functions after 4 weeks of rTMS (10 Hz on DLPFC). Positive effects started after 4 weeks of rTMS treatment and were still durable at 12 weeks of follow-up [30].
Other treatments
Inel Manav and colleagues evaluated the effect of reminiscence therapy on apathy and cognitive performance in a cohort of 72 mild AD subjects. The protocol involved internet-based videos for 60 min once a week for 3 months and resulted in improving both apathy levels and cognitive functioning [36]. A pilot study showed the positive effects of 10 weeks of horticultural therapy on apathy, cognitive, and functional abilities in 32 AD subjects. Over the course of 10 weeks of activities, a statistically significant reduction in apathy was observed in the experimental group [31].
AD—neurological correlates of apathy
Four magnetic resonance imaging (MRI) studies identified the brain regions associated with apathy in subjects with AD. In patients with moderate levels of apathy, Huey and colleagues found a greater degree of atrophy in ventromedial and ventrolateral prefrontal cortex (PFC), posterior cingulate cortex (PCC), and the superior temporal sulcus. These regions entailed brain networks responsible for arousal, threat response, and reward processing [24]. Differently, a study in patients with moderate to severe AD and with moderate apathy, performed using tensor imaging (DTI), showed bilateral damage associated with the severity of apathy in the corpus callosum and internal capsule [37]. Wei and colleagues found specific grey matter (GM) atrophy for each apathy subdomain. Emotional apathy involved cerebellum, ventral PFC, and the amygdala; executive apathy entailed left orbito-frontal cortex (OFC), bilateral frontal pole, lateral temporal regions including the right middle temporal gyrus and temporal pole, as well as the left supramarginal and angular gyri; initiation apathy involved right medial PFC, left frontal pole, OFC, the right paracingulate, and anterior cingulate cortex (ACC) [22]. Kumfor and colleagues identified three different apathy domains through NPI, Cambridge Behavioral Inventory (CBI-R), and Disability and Dementia scale (DAD). For affective apathy, GM atrophy was detected in the left temporal poles, extending into the bilateral OFC, subcallosal cortex, and bilateral insula; behavioral apathy in the frontal cortex and subcortical areas, including the left caudate, extending into the nucleus accumbens, the right precentral gyrus, and the cerebellum; cognitive apathy in left orbitofrontal and subcallosal regions, extending dorsally into the medial PFC, anterior cingulate and superior frontal gyrus, inferior temporal gyrus, and posterior cingulate cortex [21].
Three studies used resting state functional MRI (rs-fMRI) to evaluate cerebral functional connectivity (FC) underlying apathy in AD. FC is a measure of how different brain regions interact with each other [54]. Buyukgok and colleagues compared 10 apathy-early-stage-AD subjects to 10 non-apathy-AD subjects and 10 cognitively normal groups and addressed a significant hypo-functioning at a trend-level in the anterior component of default mode network (DMN), specifically in the preangular ACC in apathy-AD subjects [44]. In contrast, another rs-fMRI study on subjects with mild to severe AD did not detect any decrease in the activity of DMN between subjects with apathy (n = 35) and subject without apathy (n = 35). Downregulation of DMN, which is a typical feature of AD, was observed only between AD and the healthy control group. Moreover, reduced connectivity between the left insula and the right superior parietal cortex and increased connectivity between DLPFC and the right superior parietal cortex emerged [41]. The different findings of the two studies may be due to several reasons. First, different characteristics of the sample and small sample size; second, different methods used to detect the networks; third, taking into account that the down-regulation is seen only at a trend-level, it may be possible that DMN does not mediate apathy in AD as hypothesized by Buyukgok and colleagues [44]. A recent study of Altunkaya and colleagues described the right inferior frontal gyrus (FG), left middle FG, and left anterior insula as critical hubs for apathy in AD [13].
Yeh and colleagues used proton magnetic resonance spectroscopy to investigate apathy-related neurochemical alterations. They explored only the frontal brain regions and discovered that each subdomain of apathy was associated with neurochemical variations in the ACC, without alterations in the OFC [26].
Jeong et al. assessed the association between regional cellular blood flow (rCBF) and neuropsychological symptoms (NPS) in early AD using single-photon emission computed tomography (SPECT) in a sample of 59 patients. Results showed apathy was associated with a decrease rCBF in prefrontal, pre/postcentral, and midbrain areas [48]. Apathy-related rCBF reduction in the midbrain may be one of the novel findings in patients with early AD. This finding supports previous evidence from preclinical and clinical studies suggesting that alterations in the midbrain structure and the dysfunction of the dopaminergic system may result in the expression of apathy.
Three studies using positron emission tomography (PET) in apathy-AD subjects showed dysfunction of ACC [12, 28], DLPFC [28], and OFC [55]. More specifically, Fernández-Matarrubia and colleagues compared the features of apathy in patients with behavioral variant of FTD (bvFTD) and AD [12]. Their study demonstrated that patients with bvFTD displayed lower metabolism in the left lateral prefrontal cortex, medial frontal/anterior cingulate, and OFC and anterior insular cortices, while patients with AD were characterized by dysfunction of medial/anterior cingulate circuit without an involvement of OFC and DLPFC [12]. According to the authors, those differences may be accountable for the greater impairment of the emotional dimension observed in apathy in bvFTD compared with AD patients [12]. On the other hand, Marshall and colleagues found an association between apathy and small tau clusters within the right ACC and DLPFC, which were more pronounced in individuals with greater amyloid burden [28]. Finally, according to Kitamura and colleagues, tau tangle formation in OFC was found in patients with apathy in AD. For this reason, the authors suggest a possible therapeutic role of novel antitau drugs on apathy; however, further neuroimaging studies are needed to monitor structural and functional changes in neuronal regions involved in apathy with and without therapeutic interventions [55].
Vascular and mixed dementia
VaD is the second most common dementia worldwide after AD, accounting for 15–20% of all cases in North America and Europe [56, 57]. Recent studies identified a continuum between VaD and AD, so that the coexistence of the two is defined as mixed dementia (MxD). Although pure AD and pure VaD can be diagnosed with good accuracy, identifying mixed forms can be challenging [58, 59]. In this review, we included 10 studies about VaD and MxD. Among them, only two analyzed the MxD subgroup [10, 60] (Table 1).
VaD—apathy prevalence
Akyol et al. estimated a prevalence of 55.2% [61]. Similarly, Santos and colleagues addressed a prevalence of 56.6% [60]. A large study by Schwertner and colleagues on 10,405 patients with dementia estimated a prevalence of 30.5% among individuals affected by VaD (n = 1.708) and 29.7% in MxD (n = 1.621) [10]. Two studies reported apathy prevalence according to VaD subtype: Bhat et al. compared large vessel disease (LVD) versus small vessel disease (SVD) (28.6%vs 54.2%) [62], while according to Manso-Calderon et al., apathy was more frequent in subcortical VaD (sVaD) than in cortical VaD (cVaD) [63]. Kazui et al. stratified the prevalence of apathy according to dementia severity, assessed through the Clinical Dementia Rating scale (CDR). For VaD, they reported 52.5% for CDR 0.5, 87.7% for CDR 1, 90.3% for CDR 2, and 91.7% for CDR 3 [64].
VaD—apathy assessment
The most used tool for apathy evaluation was NPI (n = 8) [10, 13, 60,61,62,63,64,65]. Three studies used the AES [13, 66, 67]. One study used both NPI and AES [13]. Tu and colleagues stratified NPI scores according to CDR in SIVD, reporting higher scores for both the symptom and the domain of apathy in more severe dementia [65]. According to Schwertner and colleagues, of the 1708 patients affected by VaD, 11.3% suffered from mild apathy (NPI 1–3), while for 19.7% apathy was clinically significant (NPI > 3). Among 1621 individuals with MxD, 12.9% suffered from mild apathy, while for patients 17.3% apathy was clinically significant [10]. Altunkaya and colleagues analyzed the prevalence of apathy subdomains, obtained by grouping the 18 items composing AES into “initiation” (factors involved in the act of beginning an action), “motivation” (the reason for acting), and “socially” (social involvement). They reported a mean total score of 45.2 ± 14.69 and a score of 0.63 ± 1.29 for “initiation,” 0.32 ± 0.97 for “motivation,” and 0.09 ± 1.05 for “socially” [13]. To analyze the behavioral pattern in apathy and compare it to depression, Saleh et al. designed an effort-based decision-making task via psych-toolbox (psychtoolbox.org) [66].
VaD—apathy treatments
No studies explored apathy treatments as primary outcomes in VaD.
VaD—neurological correlates of apathy
Seven studies used MRI to evaluate ischemic brain damage and structural and functional variations. Tu and colleagues and Saleh and colleagues analyzed DTI parameters like fractional anisotropy (FA) and mean diffusivity (MD) [65, 66]. Altunkaya et al. performed brain mapping using rs-fMRI to evaluate FC between four different resting state networks (RSNs), which are areas of the brain showing synchronous activity at rs-fMRI, in patients with SIVD and AD [13]. Akyol et al. investigated the relation between apathy and basic and instrumental activities of daily living. According to their study, cognitive and functional decline were risk factors associated with apathy, while no statistically significant relationship was found between age, gender, and apathy [61]. According to Altunkaya et al., the volume of white matter (WM) hyperintensities (WMH), dementia staging, and Beck Depression Inventory (BDI) were significant clinical predictors of apathy [13]. Saleh et al. found that in patients with apathy, the main criterion driving decision-making was reward magnitude, as they were less responsive to low rewards and high efforts. In apathy, there was a reduction of drift rate to the decision parameter (the rate of evidence accumulation), and this change positively correlated with WM alterations. This means that patients with apathy spent less time accumulating evidence before taking a decision, and action was rejected in a shorter time, as the reward was less appealing [66]. Tu et al. found that disruption of the right superior longitudinal fasciculus predicted apathy [65]. Tay et al. showed an impaired connectivity in premotor and cingulate regions [67].
Parkinson’s disease
Parkinson’s disease (PD) is caused by degeneration of dopaminergic neurons of the substantia nigra and striatum and manifests with cardinal motor symptoms (bradykinesia, rigidity, tremor, gait/postural instability), as well as non-motor symptoms, including NPSs and autonomic dysfunction [68]. Apathy, together with depression, is one of the most common NPSs both in PD and PDD [15, 16]. We found ten studies addressing apathy in PDD. All of them addressed apathy and common NPSs in PDD, and none of them exclusively investigated apathy. A summary of studies addressing apathy in PDD included in the review is shown in Table 2.
PD and PDD—apathy prevalence
According to Modreanu and colleagues, the prevalence of moderate-to-severe apathy was 28–40% in PD and 29–52% in PDD [11]. Two studies reported a percentage of 77% [16] and 97.5% [15] in PDD.
PD and PDD—apathy assessment
Three studies used NPI [16, 69, 70]. Nine studies used apathy-specific screening tools, and among them, the Starkstein’s Apathy Scale (AS) was the most frequently used [11, 68, 71, 72]. Two studies used the AES [15, 73]. One study used the Frontal Systems Behavior Scale—Apathy subscale [70]. Studies using NPI showed lower prevalence than those using apathy-specific scales. On the other hand, Moretti and colleagues used both AES clinician and caregiver versions and addressed the highest rates of apathy [16]. Campbell et al. used a general screening tool for psychiatric functions and an apathy-specific tool; however, the authors did not report the scores [70]. Bugalho and colleagues reported a significant worsening of apathy during a follow-up period of 4 years [72], while Horne et al. did not observe significant differences during the same interval [69].
According to three studies, in patients with PDD, apathy was more severe than in subjects with PD-mild cognitive impairment (PD-MCI) and cognitively normal PD patients (PD-CN) [11, 68, 73]. In contrast, Barbosa and colleagues found similar scores among PD-CN, PD-MCI, and PDD [71], and Camargo and colleagues did not observe differences in severity in PD subjects with and without dementia [15]. Interestingly, Barbosa and colleagues found different scores among participants with and without subjective cognitive impairment (SCI). Additionally, both PD and PD-MCI subjects with SCI reported higher apathy scores than same-category participants not addressing SCI [71]. No association was found between baseline apathy measures and future dementia risk [15, 69], as well as between demographic characteristics (such as age and gender) and levels of apathy. Patients progressing to dementia had significantly greater proportions of moderate-to-severe depression, visual hallucinations, memory complaints, and non-motor predominance at baseline compared to those remaining dementia free [11].
PD and PDD—apathy treatments
Moretti and colleagues conducted an RCT on the effect of rivastigmine on apathy and addressed apathy as the most constant NPS in PDD. Patients first received a 9.5 mg/24-h dose of transdermal rivastigmine for 3 months. Therefore, a maintenance dose of rivastigmine was administered for 12 months. The first evaluation reported that 77% of patients experienced apathy, with a serious impact on daily life and with severe relevance to caregivers. After 6 months, apathy decreased in prevalence (62%), but still severely affected daily living. After 12 months, the levels of apathy increased up to 69%, while other NPS (irritability, disinhibition, and euphoria) improved [16]. Camargo et al. did not evidence a correlation between disease duration or duration of levodopa therapy and depression or apathy. However, they indicated a correlation between motor changes and NPS. More specifically, depression scores were greater in patients with more severe motor impairment, but apathy scores did not correlate with worsening motor symptoms [15].
PD and PDD—Neurological correlates of apathy
No studies exploring neural correlates of apathy in PD or PDD as a primary outcome were found.
Frontotemporal dementia
FTD is a group of neurodegenerative diseases characterized by four clinical variants distinguished by early and predominant symptoms: bvFTD, PPA, semantic variant PPA (svPPA), and nonfluent variant PPA (nfvPPA) [74]. bvFTD is the most common subtype and presents with a heterogeneous combination of socio-affective symptoms and executive deficits [75, 76]. In 2011, the consensus diagnostic criteria for bvFTD proposed six core features of the disease, namely, apathy, disinhibition, loss of empathy, change in eating behavior, stereotypical behavior, and executive dysfunction [75]. A summary of studies addressing apathy in FTD included in the review is shown in Table 3.
FTD—apathy prevalence
The prevalence of apathy in FTD was 54.8–88.0%. Apathy showed the highest reported frequency in FTD including bvFTD in ten studies [12, 21, 23, 76,77,78,79,80,81,82], while only two studies addressed PPA [76, 78]. In studies comparing FTD with AD, FTD patients had a higher prevalence of apathy [12, 21] and higher apathy scores [83, 84] than AD patients. Only one study highlighted a higher prevalence of apathy in patients with bvFTD in its early stage [82].
FTD—apathy assessment
Eight studies used NPI [21, 76, 78, 80, 82, 84,85,86], and five studies used AES [14, 23, 78, 81]. One study used LARS [12], three used CBI-R [21, 76, 77], two used the Motivation and Energy Inventory (MEI) [76, 78], three used the Snaith-Hamilton Pleasure Scale (SHAPS) [76, 78, 79], one used DAD [21], two used the Social and Emotional Assessment (Mini-SEA) [82, 87], one used AS [87], DAS was used in four studies [23, 79, 83, 88], one used the goal-directed behaviors (GDB) [81], and one used FBI [89]. Interestingly, one of the studies investigating bvFTD phenotypes related to WM changes found discordant scores on several apathy administration tests [78].
FTD—apathy treatments
None of the selected articles proposed pharmacological treatments for apathy. Godefroy et al. used a remote monitoring system named ECOCAPTURE@HOME to assess the evolution of apathy in individuals with dementia and its associated impact on their caregivers. They recruited 60 dyads of patients and caregivers (20 bvFTD, 20 AD, and 20 healthy control). Each dyad was monitored through a multisensory wearable bracelet and questionnaires on a smartphone application for 28 consecutive days. Building on the knowledge acquired through this first ECOCAPTURE@HOME study, they expected that the following phase of such a process will be to test a machine learning system, which could automatically estimate the behavioral markers of apathy and the associated caregiver’s perception of the dyad’s status using solely passive data from sensors [81].
FTD—neurological correlates of apathy
Included studies revealed that different clinical phenotypes of FTD have different profiles of functional decline and distinct patterns of associated cortical changes. Seven studies used MRI [14, 21, 77,78,79, 84, 85, 90]. One study used PET [12], and another one used SPECT [80]. In one study, 88 patients with bvFTD were included in a cluster analysis focusing on levels of disinhibition and apathy. Four phenotypic subgroups were identified in this study: primary severe apathy (n = 26), severe apathy and disinhibition (n = 26), mild apathy and disinhibition (n = 27), and primary severe disinhibition (n = 9). Results showed that apathy scores were associated with increased atrophy in the insula, inferior frontal, and anterior temporal cortex. Patients with severe apathy phenotypes were more functionally impaired with more extensive brain atrophy than those with mild apathy or primary severe disinhibition [77]. In another study, WM correlates of apathy and impulsivity were identified in the major syndromes associated with frontotemporal lobar degeneration, using diffusion-weighted imaging. The results highlighted three components associated with significant WM tract abnormalities: (1) care-rated change in daily skills, (2) changes in self-care, and (3) motivation correlated with widespread changes in dorsal frontoparietal and corticospinal tracts. In the neuropsychological tests of cognitive control, reflection impulsivity, and reward responsiveness were associated with focal changes in the right frontal lobe and supplementary motor area [78]. Another study showed that bvFTD subjects had greater impairment of emotional apathy and self-awareness than the AD sample. Such differences in the qualitative aspects of apathy appear to be associated with different functional and neuroanatomical substrates, as shown by FDG-PET imaging analysis of left lateral prefrontal, medial frontal/anterior cingulate, lateral insular cortex, and lateral OFC in bvFTD and right ACC [12]. Furthermore, neuroimaging results showed that among dementia syndromes (bFTD and AD), greater affective apathy was associated with reduced GM intensity in the ventral PFC and cognitive apathy with dorsal PFC. The presence of behavioral apathy was associated with the degradation of subcortical regions, including the caudate region of the basal ganglia, as well as the premotor cortex and cerebellum [21]. Moreover, they found that apathy progressed significantly in pre-symptomatic carriers of FTD-associated mutations and those individual differences in apathy at baseline predicted the severity of progressive deterioration in digit symbol test performance over time. In pre-symptomatic carriers, progression of apathy over 2 years was associated with baseline frontal lobe and cingulate gyrus atrophy. In contrast, subclinical cognitive impairments did not predict the worsening of apathy [90]. Another study investigated the topographic feature of the rCBF network in bvFTD patients with and without apathy. They found that both bvFTD groups retained the global function and characteristics typical of small mundanity but showed the loss of hubs distributed mainly in the PFC. Compared with the bvFTD no-apathy group, the bvFTD apathy group showed further loss of hub in the frontostriatal circuit, but recruited hubs in the angular gyrus, precuneus, and PCC. Overall, their results support previous findings from other neuroimaging modalities, in which apathy was related to frontostriatal circuit disruption in bvFTD [80]. Concerning cognitive apathy, an MRI study demonstrated that the presence of a deficit in planning ability is a predictor for a worsening in cognitive decline in both bvFTD and AD and it shares similar pathways with apathy. More precisely, alterations in the lateral and medial prefrontal and lateral temporal cortices as well as subcortical regions including the hippocampus, striatum, and thalamus were detected in both patients with apathy affected by AD and bvFTD [88]. Finally, O’Connor et al. evaluated the importance of considering anhedonia as a primary presenting feature of bvFTD and semantic dementia, with neural drivers distinct from those of apathy or depression. Overall, the results underscore the importance of apathy in functional impairment, highlighting the role of the right temporal region in disinhibition [77]. Lansdall and colleagues also suggested that apathy and impulsivity are correlated with WM tract abnormalities in the major syndromes associated with frontotemporal lobar degeneration, while daily skills, self-care, and motivation were probably related to diffuse changes in the frontoparietal and dorsal corticospinal tracts [78].
Discussion
Prevalence of apathy in different forms of dementia
Apathy was shown to have broad prevalence intervals in all types of dementia (26–82% for AD, 28.6–91.7 for VaD, 29–97.5% for PDD, and 54.8–88.0 for FTD). One possible explanation for the ambiguous prevalence is the use of multiple tools to quantify apathy and dementia severity. Most studies assessed the severity of dementia using MMSE, which may be not sufficiently accurate in moderate-to-severe dementia [91]. Nonetheless, while apathy appears to be more often connected with later stages of dementia, previous studies demonstrated that it could be prodromal for executive functions deterioration and also associated to an increased risk of developing cognitive decline in community-dwelling people [92]. Moreover, although apathy underlies similar symptoms to other disorders, such as depression [93], agreement on a standardized diagnostic system has not been established yet. Finally, except for a few research [14,15,16], all the included studies did not have apathy as their primary outcome. No studies investigating predictive risk factors for dementia primarily investigated the role of apathy, as opposed to depression or to other behavioral disorders. This has made it even more complex to outline a clear profile of apathy in relation to patients with different forms of dementia, although apathy is extremely related to these conditions.
Apathy assessment in different forms of dementia
The use of multiple diagnostic techniques for assessing apathy may explain the differences in outcomes. Several studies employed generic scales, such as the NPI, which might lead to inaccurate identification. Apathy is frequently underestimated and misdiagnosed as depression [4]. Due to the necessity for specialist training, apathy-specific measures like the AES are seldom used in clinical practice. Apathy has been discovered as a predictive biomarker for the likelihood of acquiring major diseases such as PD, cognitive decline, and severe dementia [94, 95]. An early diagnosis of apathy could help clinicians to detect dementia in earlier stages, as well as prevent frailty [96] or a misdiagnosis of depression. For these reasons, a more specific test appears to be important in assessing therapy response across multiple domains [97]. Given the prevalence of several phenotypes of apathy, the NPI appears to be a deficient scale in clinical practice for diagnosing apathy, whereas AES [98], DAS, or LARS appeared more appropriate to investigate the domains of apathy. More specifically, a systematic review reported that DAIR and AES clinician versions for AD, and LARS for PD, have the highest methodological quality and psychometric properties among the other validated apathy scales [99]. Correct diagnosis of apathy has substantial consequences for caregiver quality of life, [16] and it is likely that caregiver distress has important implications on the perception of the apathetic behavior of their assisted. Thus, employing a unique neuropsychological instrument and distinguishing scales administered by caregivers from those administered by clinicians may be relevant in future research to show possible variations in apathy identification and quantification due to the type of clinical scale.
Apathy treatments in different forms of dementia
Despite a recent increased research interest in apathy in dementia, the number of RCT for pharmacological treatments is poor. Methylphenidate produced the most meaningful findings for apathy in AD, as it improved different apathy domains. However, included studies reported discordant results on cognitive performance and caregiver distress [45, 51]. However, in these circumstances, the inclusion of non-apathy-specific testing may have a detrimental impact on the study’s results. rTMS paired with cognitive training was found to significantly enhance apathy as well as cognitive functioning among non-pharmacological therapies in AD [40]. However, as noted by a systematic review by Theleritis and colleagues, apathetic patients with dementia may benefit from personalized treatments combining both pharmacological and non-pharmacological interventions, including lifestyle changes and environmental adjustments [100].
Neurological correlates of apathy in different forms of dementia
The neurological basis of apathy is thought to be a disruption in networks controlling goal-directed behavior. Although the PFC is one of the key regions engaged in these tasks [4], changes in other brain regions might result in distinct phenotypes of apathy [101]. Recently, a new categorization of apathy was developed based on clinical findings of individuals with damage to PFC and basal ganglia [102]. Emotional apathy seems to be due to lesions in the connection between ventral-striatum fibers and orbitomedial PFC; cognitive apathy seems to involve neural connections between lateral PFC and dorsal caudate nuclei; auto-activation apathy seems to be due caused by bilateral lesions in limbic and cognitive territories in basal ganglia (internal globus pallidus and bilateral paramedian thalamus) and dorso-medial part of PFC. The finding of the neurological mechanisms underlying these illnesses is intimately connected to the function of apathy as a risk factor for neurodegenerative diseases. Neuroimaging studies in AD identified brain areas primarily related with apathy; nevertheless, even in this situation, the multiple neuropsychological tools employed to quantify apathy degree resulted in diverse and sometimes contradictory results. Indeed, Huey et al. using NPI found a greater degree of atrophy in ventromedial and ventrolateral PFC, posterior cingulate cortex, and the superior temporal sulcus [24]. Instead, Aguera-Ortiz L. et al., using the Apathy in Dementia Nursing Home Version Scale, addressed bilateral damage of the corpus callosum and internal capsule [37]. Similarly, to evaluate brain atrophy related to different apathy domains, Wei et al. applied the DSA [22], while Kumfor et al. used NPI, CBI-R, and DAD [9]. In the first study, they found GM atrophy along the brain regions implied in emotional apathy, executive apathy, and initiation apathy [22]. Conversely, in the second study, GM atrophy was detected in affective apathy, behavioral apathy, and cognitive apathy. These results demonstrated that the use of different neuropsychological tests may lead to heterogeneous and misleading data; therefore, an apathy-specific tool is recommended. Studies investigating the neuroanatomical substrate of apathy in AD and VaD supported the disruption of pathways connecting brain regions implicated in effort-based decision-making (disconnection theory) as the neurological substrate of apathy [13, 65,66,67]. In comparison to AD patients, FTD patients had a more widespread emotional deficiency, which was related with decreased self-awareness. According to an FDG-PET investigation, changes in qualitative characteristics of apathy appear to be related with several functional and neuroanatomical substrates [12]. Another neuroimaging study found that in both AD and bvFTD, affective apathy was associated with reduced GM intensity in the ventral PFC, cognitive apathy with dorsal PFC, and behavioral apathy with degradation of subcortical regions, premotor cortex, and cerebellum WM correlates of apathy and impulsivity were related with frontotemporal lobar degeneration in FTD using DTI. These WM anomalies, which were linked to alterations in daily skills, self-care, and motivation, were shown to be associated to changes in the dorsal frontoparietal and corticospinal circuits. The neuropsychological tests, on the other hand, yielded different results, linking these behavioral changes to alterations in the right frontal lobe and pre-supplementary motor area [78]. In conclusion, in both AD and VaD, apathy increases as dementia and WM atrophy progress [64, 103]. In FTD, the role of the right temporal region in disinhibition has been consistently highlighted, together with the correlation of apathy with WM tract abnormalities [77]. No data on neuroimaging correlates have been reported for PDD.
Conclusions
Despite its high frequency in neurodegenerative disorders, apathy is a neurobehavioral condition that is too often misdiagnosed. This review highlights that the appropriate diagnostic methods are still not employed in both clinical practice and research, as well as a paucity of studies that examine apathy as a primary outcome. Inadequate assessment leads to a lack of evidence on appropriate treatments for this disease. Possible therapeutic strategies to modulate this condition could be neurostimulation or cognitive training. However, agreement on the various assessment measures should be reached in order to test and reduce discrepancies between clinical studies. As far as we know, this is the first review that reports the literature on apathy based on the most common dementias. As the main limitation, the present study reflects the findings of a narrative review.
Data availability
No new data were created or analysed in this study. Data sharing is not applicable to this article.
References
Gracia-Garcia P, Modrego P, Lobo A (2021) Apathy and neurocognitive correlates: review from the perspective of “precision psychiatry.” Curr Opin Psychiatry 34(2):193–198. https://doi.org/10.1097/YCO.0000000000000677
Parrotta I, De Mauleon A, Abdeljalil AB, De Souto Barreto P, Lethin C, Veerbek H et al (2020) Depression in people with dementia and caregiver outcomes: results from the European right time place care study. J Am Med Dir Assoc. 21(6):872–8.e1. https://doi.org/10.1016/j.jamda.2020.02.023
Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS et al (2008) Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 65(10):1193–1198. https://doi.org/10.1001/archpsyc.65.10.1193
Levy R, Dubois B (2006) Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 16(7):916–928. https://doi.org/10.1093/cercor/bhj043
Raimo S, Santangelo G, D’Iorio A, Trojano L, Grossi D (2019) Neural correlates of apathy in patients with neurodegenerative disorders: an activation likelihood estimation (ALE) meta-analysis. Brain Imaging Behav 13(6):1815–1834. https://doi.org/10.1007/s11682-018-9959-0
Stella F, de Andrade LP, Vital TM, Coelho FGM, Nascimento CMC, Hernandez SSS (2010) Apathy in Alzheimer’s disease: contribution to a clinical view on progression of dementia. Dement Neuropsychol 4(3):188–193. https://doi.org/10.1590/S1980-57642010DN40300007
Deardorff WJ, Grossberg GT (2019) Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. Handb Clin Neurol 165:5–32. https://doi.org/10.1016/B978-0-444-64012-3.00002-2
Tsai CF, Hwang WS, Lee JJ, Wang WF, Huang LC, Huang LK et al (2021) Predictors of caregiver burden in aged caregivers of demented older patients. BMC Geriatr 21(1):59. https://doi.org/10.1186/s12877-021-02007-1
Kumfor F, Halliday GM, Piguet O (2017) Clinical aspects of Alzheimer’s disease. Adv Neurobiol 15:31–53. https://doi.org/10.1007/978-3-319-57193-5_2
Schwertner E, Pereira JB, Xu H, Secnik J, Winblad B, Eriksdotter M et al (2022) Behavioral and psychological symptoms of dementia in different dementia disorders: a large-scale study of 10,000 individuals. J Alzheimers Dis 87(3):1307–1318. https://doi.org/10.3233/JAD-215198
Modreanu R, Cerquera SC, Marti MJ, Rios J, Sanchez-Gomez A, Camara A et al (2017) Cross-sectional and longitudinal associations of motor fluctuations and non-motor predominance with cerebrospinal tau and Abeta as well as dementia-risk in Parkinson’s disease. J Neurol Sci 373:223–229. https://doi.org/10.1016/j.jns.2016.12.064
Fernandez-Matarrubia M, Matias-Guiu JA, Cabrera-Martin MN, Moreno-Ramos T, Valles-Salgado M, Carreras JL et al (2018) Different apathy clinical profile and neural correlates in behavioral variant frontotemporal dementia and Alzheimer’s disease. Int J Geriatr Psychiatry 33(1):141–150. https://doi.org/10.1002/gps.4695
Altunkaya S, Huang SM, Hsu YH, Yang JJ, Lin CY, Kuo LW et al (2021) Dissociable functional brain networks associated with apathy in subcortical ischemic vascular disease and Alzheimer’s disease. Front Aging Neurosci 13:717037. https://doi.org/10.3389/fnagi.2021.717037
Alfano V, Longarzo M, Mele G, Esposito M, Aiello M, Salvatore M et al (2021) Identifying a common functional framework for apathy large-scale brain network. J Pers Med 11(7). https://doi.org/10.3390/jpm11070679
Camargo CHF, Serpa RA, Jobbins VA, Berbetz FA, Sabatini JS (2018) Differentiating between apathy and depression in patients with Parkinson disease dementia. Am J Alzheimers Dis Other Demen 33(1):30–34. https://doi.org/10.1177/1533317517728333
Moretti R, Caruso P, Dal Ben M (2017) Rivastigmine as a symptomatic treatment for apathy in Parkinson’s dementia complex: new aspects for this riddle. Parkinsons Dis 2017:6219851. https://doi.org/10.1155/2017/6219851
Drijgers RL, Aalten P, Winogrodzka A, Verhey FR, Leentjens AF (2009) Pharmacological treatment of apathy in neurodegenerative diseases: a systematic review. Dement Geriatr Cogn Disord 28(1):13–22. https://doi.org/10.1159/000228840
Robert PH, Mulin E, Mallea P, David R (2010) Review: Apathy diagnosis, assessment, and treatment in Alzheimer’s disease. CNS Neurosci Ther 16(5):263–271. https://doi.org/10.1111/j.1755-5949.2009.00132.x
Sepehry AA, Sarai M, Hsiung GR (2017) Pharmacological therapy for apathy in Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci 44(3):267–275. https://doi.org/10.1017/cjn.2016.426
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Kumfor F, Zhen A, Hodges JR, Piguet O, Irish M (2018) Apathy in Alzheimer’ disease and frontotemporal dementia: distinct clinical profiles and neural correlates. Cortex 103:350–359. https://doi.org/10.1016/j.cortex.2018.03.019
Wei G, Irish M, Hodges JR, Piguet O, Kumfor F (2020) Disease-specific profiles of apathy in Alzheimer’s disease and behavioural-variant frontotemporal dementia differ across the disease course. J Neurol 267(4):1086–1096. https://doi.org/10.1007/s00415-019-09679-1
Radakovic R, Colville S, Cranley D, Starr JM, Pal S, Abrahams S (2021) Multidimensional apathy in behavioral variant frontotemporal dementia, primary progressive aphasia, and Alzheimer disease. J Geriatr Psychiatry Neurol 34(5):349–356. https://doi.org/10.1177/0891988720924716
Huey ED, Lee S, Cheran G, Grafman J, Devanand DP (2017) Alzheimer’s disease neuroimaging I. Brain regions involved in arousal and reward processing are associated with apathy in Alzheimer’s disease and frontotemporal dementia. J Alzheimers Dis. 55(2):551–8. https://doi.org/10.3233/JAD-160107
Jacus JP (2017) Awareness, apathy, and depression in Alzheimer’s disease and mild cognitive impairment. Brain Behav 7(4):e00661. https://doi.org/10.1002/brb3.661
Yeh YC, Li CW, Kuo YT, Huang MF, Liu TL, Jaw TS et al (2018) Association between altered neurochemical metabolites and apathy in patients with Alzheimer’s disease. Int Psychogeriatr 30(5):761–768. https://doi.org/10.1017/S1041610217002381
Padala PR, Padala KP, Lensing SY, Jackson AN, Hunter CR, Parkes CM et al (2018) Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: a double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 261:312–318. https://doi.org/10.1016/j.psychres.2017.12.063
Marshall GA, Gatchel JR, Donovan NJ, Muniz MC, Schultz AP, Becker JA et al (2019) Regional tau correlates of instrumental activities of daily living and apathy in mild cognitive impairment and Alzheimer’s disease dementia. J Alzheimers Dis 67(2):757–768. https://doi.org/10.3233/JAD-170578
Yu SY, Lian TH, Guo P, Li LX, Ding DY, Li DN et al (2020) Correlations of apathy with clinical symptoms of Alzheimer’s disease and olfactory dysfunctions: a cross-sectional study. BMC Neurol 20(1):416. https://doi.org/10.1186/s12883-020-01978-9
Padala PR, Boozer EM, Lensing SY, Parkes CM, Hunter CR, Dennis RA et al (2020) Neuromodulation for apathy in Alzheimer’s disease: a double-blind, randomized, sham-controlled pilot study. J Alzheimers Dis 77(4):1483–1493. https://doi.org/10.3233/JAD-200640
Yang Y, Kwan RYC, Zhai HM, Xiong Y, Zhao T, Fang KL et al (2022) Effect of horticultural therapy on apathy in nursing home residents with dementia: a pilot randomized controlled trial. Aging Ment Health 26(4):745–753. https://doi.org/10.1080/13607863.2021.1907304
Scherer RW, Drye L, Mintzer J, Lanctot K, Rosenberg P, Herrmann N et al (2018) The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials 19(1):46. https://doi.org/10.1186/s13063-017-2406-5
Lanctot KL, Scherer RW, Li A, Vieira D, Coulibaly H, Rosenberg PB et al (2021) Measuring apathy in Alzheimer’s disease in the Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): a comparison of instruments. Am J Geriatr Psychiatry 29(1):81–89. https://doi.org/10.1016/j.jagp.2020.05.020
Radakovic R, McGrory S, Chandran S, Swingler R, Pal S, Stephenson L et al (2020) The brief dimensional apathy scale: a short clinical assessment of apathy. Clin Neuropsychol 34(2):423–435. https://doi.org/10.1080/13854046.2019.1621382
Radakovic R, Davenport R, Starr JM, Abrahams S (2018) Apathy dimensions in Parkinson’s disease. Int J Geriatr Psychiatry 33(1):151–158. https://doi.org/10.1002/gps.4697
Inel Manav A, Simsek N (2019) The effect of reminiscence therapy with internet-based videos on cognitive status and apathy of older people with mild dementia. J Geriatr Psychiatry Neurol 32(2):104–113. https://doi.org/10.1177/0891988718819864
Aguera-Ortiz L, Hernandez-Tamames JA, Martinez-Martin P, Cruz-Orduna I, Pajares G, Lopez-Alvarez J et al (2017) Structural correlates of apathy in Alzheimer’s disease: a multimodal MRI study. Int J Geriatr Psychiatry 32(8):922–930. https://doi.org/10.1002/gps.4548
Ohsawa M, Tanaka Y, Ehara Y, Makita S, Onaka K (2017) A possibility of simultaneous treatment with the multicomponent drug, Ninjin’yoeito, for anorexia, apathy, and cognitive dysfunction in frail Alzheimer’s disease Patients: An Open-Label Pilot Study. J Alzheimers Dis Rep 1(1):229–235. https://doi.org/10.3233/ADR-170026
Gatchel JR, Donovan NJ, Locascio JJ, Becker JA, Rentz DM, Sperling RA et al (2017) Regional 18F-fluorodeoxyglucose hypometabolism is associated with higher apathy scores over time in early Alzheimer disease. Am J Geriatr Psychiatry 25(7):683–693. https://doi.org/10.1016/j.jagp.2016.12.017
Nguyen JP, Suarez A, Kemoun G, Meignier M, Le Saout E, Damier P et al (2017) Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease. Neurophysiol Clin 47(1):47–53. https://doi.org/10.1016/j.neucli.2017.01.001
Jones SA, De Marco M, Manca R, Bell SM, Blackburn DJ, Wilkinson ID et al (2019) Altered frontal and insular functional connectivity as pivotal mechanisms for apathy in Alzheimer’s disease. Cortex 119:100–110. https://doi.org/10.1016/j.cortex.2019.04.008
Zhu CW, Grossman HT, Sano M (2019) Why do they just sit? Apathy as a core symptom of Alzheimer disease. Am J Geriatr Psychiatry 27(4):395–405. https://doi.org/10.1016/j.jagp.2018.12.013
Valotassiou V, Angelidis G, Psimadas D, Tsougos I, Georgoulias P (2021) In the era of FDG PET, is it time for brain perfusion SPECT to gain a place in Alzheimer’s disease imaging biomarkers? Eur J Nucl Med Mol Imaging 48(4):969–971. https://doi.org/10.1007/s00259-020-05077-2
Buyukgok D, Bayraktaroglu Z, Buker HS, Kulaksizoglu MIB, Gurvit IH (2020) Resting-state fMRI analysis in apathetic Alzheimer’s disease. Diagn Interv Radiol 26(4):363–369. https://doi.org/10.5152/dir.2019.19445
Mintzer J, Lanctot KL, Scherer RW, Rosenberg PB, Herrmann N, van Dyck CH et al (2021) Effect of methylphenidate on apathy in patients with Alzheimer disease: The ADMET 2 randomized clinical trial. JAMA Neurol 78(11):1324–1332. https://doi.org/10.1001/jamaneurol.2021.3356
Grossman HT, Sano M, Aloysi A, Elder GA, Neugroschl J, Schimming C et al (2021) Prevalent, persistent, and impairing: longitudinal course and impact of apathy in Alzheimer’s disease. Alzheimers Dement (Amst) 13(1):e12169. https://doi.org/10.1002/dad2.12169
Altomari N, Bruno F, Lagana V, Smirne N, Colao R, Curcio S et al (2022) A comparison of behavioral and Psychological Symptoms of dementia (BPSD) and BPSD sub-syndromes in early-onset and late-onset Alzheimer’s disease. J Alzheimers Dis 85(2):691–699. https://doi.org/10.3233/JAD-215061
Jeong H, Kang I, Park JS, Na SH, Kim S, Yoon S et al (2022) Regional cerebral blood flow correlates of neuropsychiatric symptom domains in early Alzheimer’s disease. Diagnostics (Basel) 12(5). https://doi.org/10.3390/diagnostics12051246
Palmieri B, Vadala M (2023) Oral THC: CBD cannabis extract in main symptoms of Alzheimer disease: agitation and weight loss. Clin Ter 174(1):53–60. https://doi.org/10.7417/CT.2023.2497
Pillai JA, Bena J, Rothenberg K, Boron B, Leverenz JB (2022) Association of variation in behavioral symptoms with initial cognitive phenotype in adults with dementia confirmed by neuropathology. JAMA Netw Open 5(3):e220729. https://doi.org/10.1001/jamanetworkopen.2022.0729
Padala PR, Padala KP, Lensing SY, Ramirez D, Monga V, Bopp MM et al (2018) Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: a double-blind, randomized, placebo-controlled trial. Am J Psychiatry 175(2):159–168. https://doi.org/10.1176/appi.ajp.2017.17030316
Takemoto M, Ohta Y, Hishikawa N, Yamashita T, Nomura E, Tsunoda K et al (2020) The efficacy of sertraline, escitalopram, and nicergoline in the treatment of depression and apathy in Alzheimer’s disease: the Okayama Depression and Apathy Project (ODAP). J Alzheimers Dis 76(2):769–772. https://doi.org/10.3233/JAD-200247
Guzman-Martinez L, Farias GA, Tapia JP, Sanchez MP, Fuentes P, Gloger S et al (2021) Interventional study to evaluate the clinical effects and safety of the nutraceutical compound BrainUp-10(R) in a cohort of patients with Alzheimer’s disease: a multicenter, randomized, double-blind, and placebo-controlled trial. J Alzheimers Dis 81(3):1231–1241. https://doi.org/10.3233/JAD-201501
Seitzman BA, Snyder AZ, Leuthardt EC, Shimony JS (2019) The state of resting state networks. Top Magn Reson Imaging 28(4):189–196. https://doi.org/10.1097/RMR.0000000000000214
Kitamura S, Shimada H, Niwa F, Endo H, Shinotoh H, Takahata K et al (2018) Tau-induced focal neurotoxicity and network disruption related to apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 89(11):1208–1214. https://doi.org/10.1136/jnnp-2018-317970
Bir SC, Khan MW, Javalkar V, Toledo EG, Kelley RE (2021) Emerging concepts in vascular dementia: a review. J Stroke Cerebrovasc Dis 30(8):105864. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105864
Wolters FJ, Ikram MA (2019) Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol 39(8):1542–1549. https://doi.org/10.1161/ATVBAHA.119.311908
Fierini F (2020) Mixed dementia: neglected clinical entity or nosographic artifice? J Neurol Sci 410:116662. https://doi.org/10.1016/j.jns.2019.116662
Zekry D, Gold G (2010) Management of mixed dementia. Drugs Aging 27(9):715–728. https://doi.org/10.2165/11538250-000000000-00000
Santos MAO, Bezerra LS, Correia CDC, Bruscky IS (2018) Neuropsychiatric symptoms in vascular dementia: epidemiologic and clinical aspects. Dement Neuropsychol 12(1):40–44. https://doi.org/10.1590/1980-57642018dn12-010006
Akyol MA, Kucukguclu O, Yener G (2020) Investigation of factors affecting apathy in three major types of dementia. Noro Psikiyatr Ars. 57(2):120–5. https://doi.org/10.29399/npa.22964
Bhat A, Biswas A, Das G, Lahiri D, Dubey S, Mukherjee A (2023) Behavioral variations among vascular cognitive impairment subtypes - a comparative study. Appl Neuropsychol Adult 30(4):439–446. https://doi.org/10.1080/23279095.2021.1954002
Manso-Calderon R, Cacabelos-Perez P, Sevillano-Garcia MD, Herrero-Prieto ME, Gonzalez-Sarmiento R (2020) The impact of vascular burden on behavioural and psychological symptoms in older adults with dementia: the BEVASDE study. Neurol Sci 41(1):165–174. https://doi.org/10.1007/s10072-019-04071-3
Kazui H, Yoshiyama K, Kanemoto H, Suzuki Y, Sato S, Hashimoto M et al (2016) Differences of behavioral and psychological symptoms of dementia in disease severity in four major dementias. PLoS One 11(8):e0161092. https://doi.org/10.1371/journal.pone.0161092
Tu MC, Huang WH, Hsu YH, Lo CP, Deng JF, Huang CF (2017) Comparison of neuropsychiatric symptoms and diffusion tensor imaging correlates among patients with subcortical ischemic vascular disease and Alzheimer’s disease. BMC Neurol 17(1):144. https://doi.org/10.1186/s12883-017-0911-5
Saleh Y, Le Heron C, Petitet P, Veldsman M, Drew D, Plant O et al (2021) Apathy in small vessel cerebrovascular disease is associated with deficits in effort-based decision making. Brain 144(4):1247–1262. https://doi.org/10.1093/brain/awab013
Tay J, Tuladhar AM, Hollocks MJ, Brookes RL, Tozer DJ, Barrick TR et al (2019) Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology 92(11):e1157–e1167. https://doi.org/10.1212/WNL.0000000000007095
Tajiri Y, Wada-Isoe K, Tanaka K, Adachi T, Hanajima R, Nakashima K (2020) A single-institution study on predictors of short-term progression from mild cognitive impairment in Parkinson’s disease to Parkinson’s disease with dementia. Yonago Acta Med 63(1):28–33. https://doi.org/10.33160/yam.2020.02.004
Horne KL, MacAskill MR, Myall DJ, Livingston L, Grenfell S, Pascoe MJ et al (2021) Neuropsychiatric symptoms are associated with dementia in Parkinson’s disease but not predictive of it. Mov Disord Clin Pract 8(3):390–399. https://doi.org/10.1002/mdc3.13151
Campbell MC, Myers PS, Weigand AJ, Foster ER, Cairns NJ, Jackson JJ et al (2020) Parkinson disease clinical subtypes: key features & clinical milestones. Ann Clin Transl Neurol 7(8):1272–1283. https://doi.org/10.1002/acn3.51102
Barbosa RP, Mendonca MD, Caetano AP, Lampreia TM, Miguel R, Bugalho PM (2019) Cognitive complaints in Parkinson’s disease patients: from subjective cognitive complaints to dementia and affective disorders. J Neural Transm (Vienna) 126(10):1329–1335. https://doi.org/10.1007/s00702-019-02042-8
Bugalho P, Ladeira F, Barbosa R, Marto JP, Borbinha C, da Conceicao L et al (2021) Progression in Parkinson’s disease: variation in motor and non-motor symptoms severity and predictors of decline in cognition, motor function, disability, and health-related quality of life as assessed by two different methods. Mov Disord Clin Pract 8(6):885–895. https://doi.org/10.1002/mdc3.13262
Wojtala J, Heber IA, Neuser P, Heller J, Kalbe E, Rehberg SP et al (2019) Cognitive decline in Parkinson’s disease: the impact of the motor phenotype on cognition. J Neurol Neurosurg Psychiatry 90(2):171–179. https://doi.org/10.1136/jnnp-2018-319008
Lanata SC, Miller BL (2016) The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry 87(5):501–511. https://doi.org/10.1136/jnnp-2015-310697
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(Pt 9):2456–2477. https://doi.org/10.1093/brain/awr179
Lansdall CJ, Coyle-Gilchrist ITS, Vazquez Rodriguez P, Wilcox A, Wehmann E, Robbins TW et al (2019) Prognostic importance of apathy in syndromes associated with frontotemporal lobar degeneration. Neurology 92(14):e1547–e1557. https://doi.org/10.1212/WNL.0000000000007249
O’Connor CM, Landin-Romero R, Clemson L, Kaizik C, Daveson N, Hodges JR et al (2017) Behavioral-variant frontotemporal dementia: distinct phenotypes with unique functional profiles. Neurology 89(6):570–577. https://doi.org/10.1212/WNL.0000000000004215
Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vazquez Rodriguez P, Wilcox A, Wehmann E et al (2018) White matter change with apathy and impulsivity in frontotemporal lobar degeneration syndromes. Neurology 90(12):e1066–e1076. https://doi.org/10.1212/WNL.0000000000005175
Shaw SR, El-Omar H, Roquet D, Hodges JR, Piguet O, Ahmed RM et al (2021) Uncovering the prevalence and neural substrates of anhedonia in frontotemporal dementia. Brain 144(5):1551–1564. https://doi.org/10.1093/brain/awab032
Zhou Z, Zheng X, Li R, Zheng Y, Jin Y, Jia S et al (2021) Alterations of cerebral blood flow network in behavioral variant frontotemporal dementia patients with and without apathy. Psychiatry Res Neuroimaging 307:111203. https://doi.org/10.1016/j.pscychresns.2020.111203
Godefroy V, Tanguy D, Bouzigues A, Sezer I, Ferrand-Verdejo J, Azuar C et al (2021) Frontotemporal dementia subtypes based on behavioral inhibition deficits. Alzheimers Dement (Amst) 13(1):e12178. https://doi.org/10.1002/dad2.12178
Musa Salech G, Lillo P, van der Hiele K, Mendez-Orellana C, Ibanez A, Slachevsky A (2021) Apathy, executive function, and emotion recognition are the main drivers of functional impairment in behavioral variant of frontotemporal dementia. Front Neurol 12:734251. https://doi.org/10.3389/fneur.2021.734251
Wong S, Wei G, Husain M, Hodges JR, Piguet O, Irish M et al (2023) Altered reward processing underpins emotional apathy in dementia. Cogn Affect Behav Neurosci 23(2):354–370. https://doi.org/10.3758/s13415-022-01048-2
Valotassiou V, Sifakis N, Tzavara C, Lykou E, Tsinia N, Kamtsadeli V et al (2022) Differences of apathy perfusion correlates between Alzheimer’s disease and frontotemporal dementia. A 99mTc-HMPAO SPECT study with automated Brodmann areas analysis. Int J Psychiatry Clin Pract 26(1):14–22. https://doi.org/10.1080/13651501.2020.1846752
Basavaraju R, Feng X, France J, Huey ED, Provenzano FA (2022) Depression is associated with preserved cortical thickness relative to apathy in frontotemporal dementia. J Geriatr Psychiatry Neurol 35(1):78–88. https://doi.org/10.1177/0891988720964258
Pengo M, Alberici A, Libri I, Benussi A, Gadola Y, Ashton NJ et al (2022) Sex influences clinical phenotype in frontotemporal dementia. Neurol Sci 43(9):5281–5287. https://doi.org/10.1007/s10072-022-06185-7
Mariano LI, Caramelli P, Guimaraes HC, Gambogi LB, Moura MVB, Yassuda MS et al (2020) Can social cognition measurements differentiate behavioral variant frontotemporal dementia from Alzheimer’s disease regardless of apathy? J Alzheimers Dis 74(3):817–827. https://doi.org/10.3233/JAD-190861
Eggins P, Wong S, Wei G, Hodges JR, Husain M, Piguet O et al (2022) A shared cognitive and neural basis underpinning cognitive apathy and planning in behavioural-variant frontotemporal dementia and Alzheimer’s disease. Cortex 154:241–253. https://doi.org/10.1016/j.cortex.2022.05.012
Chu M, Jiang D, Liu L, Nie B, Rosa-Neto P, Chen K et al (2023) Clinical relevance of disrupted topological organization of anatomical connectivity in behavioral variant frontotemporal dementia. Neurobiol Aging 124:29–38. https://doi.org/10.1016/j.neurobiolaging.2023.01.004
Malpetti M, Jones PS, Tsvetanov KA, Rittman T, van Swieten JC, Borroni B et al (2021) Apathy in presymptomatic genetic frontotemporal dementia predicts cognitive decline and is driven by structural brain changes. Alzheimers Dement 17(6):969–983. https://doi.org/10.1002/alz.12252
Leung DKY, Chan WC, Spector A, Wong GHY (2021) Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta-analysis. Int J Geriatr Psychiatry 36(9):1330–1344. https://doi.org/10.1002/gps.5556
Bock MA, Bahorik A, Brenowitz WD, Yaffe K (2020) Apathy and risk of probable incident dementia among community-dwelling older adults. Neurology 95(24):e3280–e3287. https://doi.org/10.1212/WNL.0000000000010951
Cohen ML, Aita S, Mari Z, Brandt J (2015) The unique and combined effects of apathy and depression on cognition in Parkinson’s disease. J Parkinsons Dis 5(2):351–359. https://doi.org/10.3233/JPD-140484
Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P (2015) Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol 14(5):518–531. https://doi.org/10.1016/S1474-4422(15)00019-8
Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M et al (2007) Epidemiology of apathy in older adults: the cache county study. Am J Geriatr Psychiatry 15(5):365–375. https://doi.org/10.1097/01.JGP.0000235689.42910.0d
Parrotta I, Maltais M, Rolland Y, Spampinato DA, Robert P, de Souto BP et al (2020) The association between apathy and frailty in older adults: a new investigation using data from the Mapt study. Aging Ment Health 24(12):1985–1989. https://doi.org/10.1080/13607863.2019.1650890
Cummings J (2020) The neuropsychiatric inventory: development and applications. J Geriatr Psychiatry Neurol 33(2):73–84. https://doi.org/10.1177/0891988719882102
Marin RS (1991) Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 3(3):243–254. https://doi.org/10.1176/jnp.3.3.243
Radakovic R, Abrahams S (2014) Developing a new apathy measurement scale: dimensional apathy scale. Psychiatry Res 219(3):658–663. https://doi.org/10.1016/j.psychres.2014.06.010
Cecchi F, Pancani S, Antonioli D, Avila L, Barilli M, Gambini M et al (2018) Predictors of recovering ambulation after hip fracture inpatient rehabilitation. BMC Geriatr 18(1):201. https://doi.org/10.1186/s12877-018-0884-2
Duffy J (2000) Apathy in neurologic disorders. Curr Psychiatry Rep 2(5):434–439. https://doi.org/10.1007/s11920-000-0029-z
Quaranta D, Marra C, Rossi C, Gainotti G, Masullo C (2012) Different apathy profile in behavioral variant of frontotemporal dementia and Alzheimer’s disease: a preliminary investigation. Curr Gerontol Geriatr Res 2012:719250. https://doi.org/10.1155/2012/719250
Tanaka H, Hashimoto M, Fukuhara R, Ishikawa T, Yatabe Y, Kaneda K et al (2015) Relationship between dementia severity and behavioural and psychological symptoms in early-onset Alzheimer’s disease. Psychogeriatrics 15(4):242–247. https://doi.org/10.1111/psyg.12108
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Writing—original draft: IP, SC, FD, MD, GG, GL, GA, NM. Writing—review and editing: IP, SC, FD, MD, GG, GL, GA, NM. Visualization: IP, SC, FD, MD, GG, GL, GA, NM. Methodology: IP, GL, GA, NM. Validation: IP, GL, GA, NM. Investigation: IP, SC, FD, MD, GG, GL, GA, NM. Supervision: IP, GL, GA, NM. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
'The authors declare no competing interests.
Ethical approval
Not required.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parrotta, I., Cacciatore, S., D’Andrea, F. et al. Prevalence, treatment, and neural correlates of apathy in different forms of dementia: a narrative review. Neurol Sci 45, 1343–1376 (2024). https://doi.org/10.1007/s10072-023-07197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07197-7